-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(9): 2952-2957
doi:10.5923/j.ajmms.20251509.23
Received: Aug. 12, 2025; Accepted: Sep. 6, 2025; Published: Sep. 20, 2025

Study of Immunohistochemical Marker Expression in Thymus and Skin: An Experimental Approach
Sapaev Zafarjon Turabaevich
Independent Researcher, at Bukhara State Medical Institute, Bukhara, Uzbekistan
Correspondence to: Sapaev Zafarjon Turabaevich, Independent Researcher, at Bukhara State Medical Institute, Bukhara, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The immune system is formed as a complex of organs and tissues that protect against both endogenous and exogenous factors. It emerged in the early stages of evolution, and its function is based on recognizing and eliminating foreign antigens, which is essential for the organism's survival. Currently, a body of evidence has been gathered confirming that the immune system ensures the body's resistance to the effects of biological factors [9,15].
Keywords: Immunohistochemical method, Thymus and skin of laboratory animals, Expression, CD138 marker, S-100 marker
Cite this paper: Sapaev Zafarjon Turabaevich, Study of Immunohistochemical Marker Expression in Thymus and Skin: An Experimental Approach, American Journal of Medicine and Medical Sciences, Vol. 15 No. 9, 2025, pp. 2952-2957. doi: 10.5923/j.ajmms.20251509.23.
Article Outline
1. Introduction
- The thymus is a paired organ located behind the breastbone, and it was named after the Greek word for the herb "thyme" because its structure resembles a thyme leaf. Since its external appearance is similar to a fork and it performs an endocrine function, it is also called the thymus gland. The thymus consists of two parts: the cortex and the medulla. The cortex is composed of 90% various lymphocytes, while the remaining 10% consists of epithelial cells and macrophages. At birth, the thymus weighs 10 g, and by the age of 10-15, it increases fourfold, reaching its maximum size. Then its involution begins, during which adipose tissue increases in the cortical and medullary layers. The thymus remains active throughout a person's life [4].Today, the skin, which is considered one of the peripheral organs of the immune system, is the largest organ of the human body. Its surface area is an average of 2.3 m², which is equal to 15-20% of a person's body weight. The skin performs functions such as acting as a barrier, an immunological organ, a sensory receptor, a regulator of salt and water metabolism, a thermoregulator, a blood depot, and a site for vitamin D synthesis [3,10].The epidermis contains Langerhans dendritic antigen-presenting cells, or epidermal macrophages (5-10%). Langerhans cells have MHC I and MHC II molecules on their surface, as well as Fc receptors for IgG. Functionally, Langerhans cells are typical macrophages belonging to the body's monocyte-macrophage system. They have phagocytic activity, bind antigens, and then migrate to regional lymph nodes, where they initiate the skin's immune responses by presenting information about the antigen to T-cells. T-lymphocytes in the epidermis, similar to those in the thymus, undergo antigen-independent differentiation. In the epidermis, lymphocytes make up 0.16% of all cells, with 90% of skin lymphocytes being T-lymphocytes. Epidermal lymphocytes are mainly represented by T-killers, while T-helpers are located in the dermis, localized around the postcapillary venules of superficial vascular plexuses. Skin lymphocytes constantly recirculate while performing their functions, and the entry of lymphocytes into the skin increases as a result of antigenic stimulation. Some of the lymphocytes remain in the skin for a long time, serving as the substrate for immunological memory [10].The effects of various pathogens that cause bacterial purulent-inflammatory diseases (PIDs) that form in the skin lead to a decrease in the functional activity of the immune system, including the thymus. To this day, the specific nature of the changes in the thymus during the formation and development of bacterial PIDs has not been fully determined.
2. Purpose of the Research
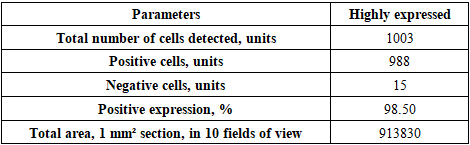
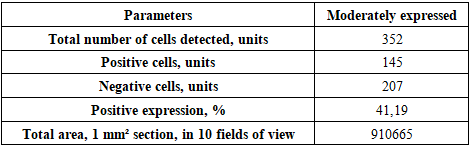
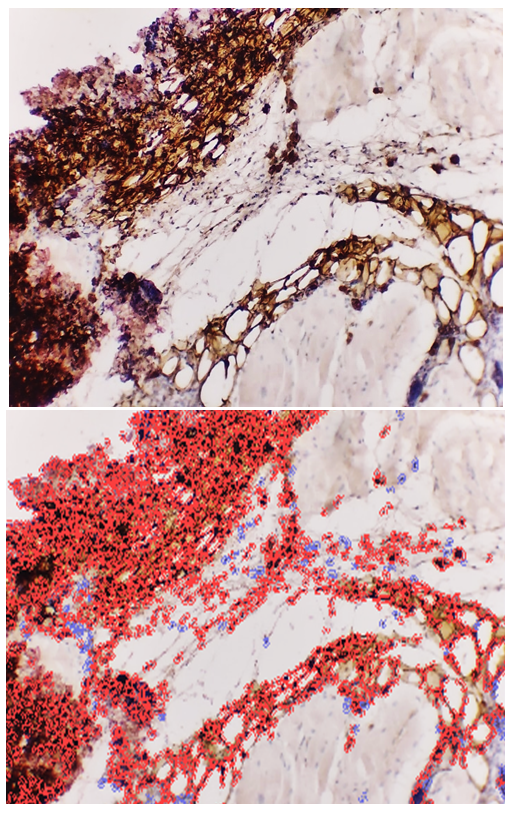
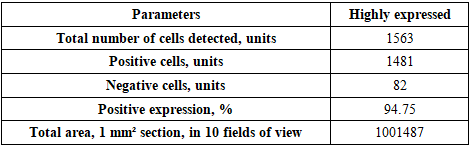
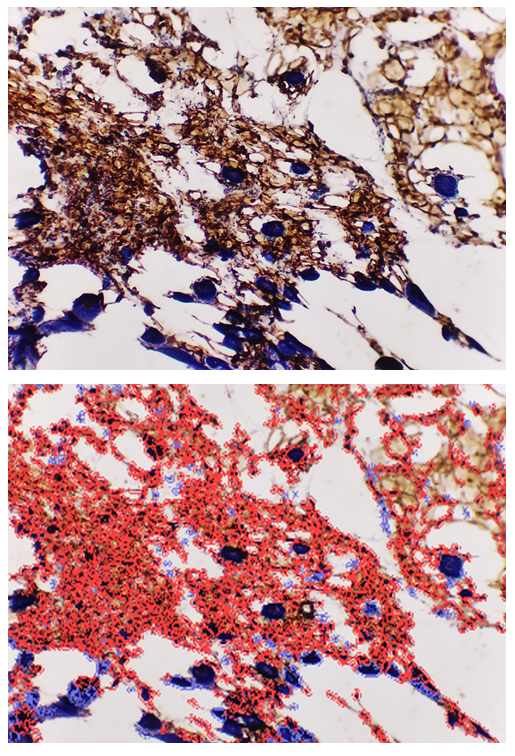
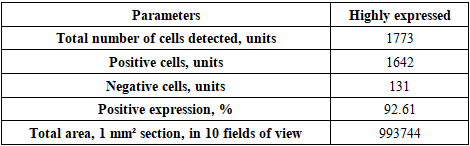
- The goal of the study is to determine the immunohistochemical expression of the CD138 marker in the thymus and the S-100 marker in the skin of laboratory animals in an experiment. It was found that in the thymus tissues of laboratory animals with purulent-inflammatory diseases (PIDs) caused by gram-positive cocci, the CD138 marker was expressed at a high level, with a positive expression of 98.50%. In PIDs caused by gram-negative bacteria, the CD138 marker was found to be expressed at a moderate level, and the positive expression parameter also decreased to 41.19%. It was proven that the level of expression in PIDs caused by gram-negative bacteria was lower compared to gram-positive cocci. In the skin tissues, the S-100 marker was expressed at a high level, with a positive expression of 94.75%. In PIDs caused by gram-negative bacteria, it was also proven that the S-100 marker was expressed at a high level in the skin tissues, and the positive expression remained high (92.61%). The IHC method also made it possible to evaluate the severity of PIDs in the context of the causative agents.
3. Materials and Methods
- The 60 male outbred white rats weighing 160-180 g were selected for the experimental studies. All laboratory animals were obtained from the same vivarium and kept in standard plastic cages under controlled conditions: 50-60% relative humidity, 19-22°C temperature, and a 12-hour light/dark cycle. The rats' diet, care, and housing followed the standards recommended by Nuraliev N.A. et al. [8]. All rules of biosafety and ethical principles for working with laboratory animals were strictly observed during the housing, euthanasia, and dissection of the animals [6,7]. The study was randomized and adhered to the principles of evidence-based medicine.Immunohistochemical research (IHC) is a microscopic tissue examination method that uses special antibodies to detect and localize the presence of antigens in tissues and cells [2,5].The results of IHC can indicate the presence or absence of specific molecules or proteins in tissues. A positive result shows the presence of the sought-after protein/molecule in the tissues, while a negative result indicates their absence. IHC is based on treating tissue sections with specific antibodies that are directed against the substance to be detected, which acts as the antigen. If the substance is present in the test material, the antibodies will bind to its specific sites, forming a complex that results in tissue staining [1,14].To perform IHC, a tissue sample is required. This sample is fixed in a fixative solution, depending on the antigen to be detected. The material is then embedded in a paraffin block and cut into thin sections (5 µm thick) using a microtome. These sections are then placed on special glass slides with an adhesive layer. After the sections are placed on the glass, deparaffinization is performed using a xylene solvent. Then, several more preparatory steps are carried out to "prepare" the antigens for reaction with the necessary antibodies, after which the IHC is performed. In the final stage, the sections are viewed under a microscope, and the tissue color is evaluated [12].Conditions for Histochemical Staining: the substance under study must be present in the histological section. Sample preparation should not affect its concentration or distribution in the tissues. The substance being studied must retain its ability to react. It must enter into a sufficiently specific qualitative reaction. The result of the histochemical reaction should be colored and visible under a light microscope. The result of the histochemical reaction must be stable and insoluble, meaning it should form a precipitate to mark the substance [1,5,14].Biochemical Markers Used: CD-138 (Cluster of Differentiation 138) is a plasma cell membrane protein not found on other immune cells. The detection of these cells marked with the CD-138 code allows for suspicion of a purulent-inflammatory process (PIP). The norm for CD138 is: 0 cells – normal, a single cell – weakly expressed, 2-3 cells – moderately expressed, more than 5 – strongly expressed. For CD138 markers, the number of positive cells was counted in 10 fields of view per 1 mm² of the section. CD138 expression was evaluated by counting positive cells in 10 fields of view at 400x magnification [13].S-100 protein is also expressed by Langerhans cells in the skin and by interdigitating reticulum cells in the paracortex of lymph nodes. The characteristics of this biomarker are: monoclonal type; PBM-2E4 (2E4) clone; S-100 binding marker; antigen S100, full-size recombinant protein; IgG2a isotype; cytoplasmic localization. The average level of S-100 protein is 10-110 ng/mL (0.01-0.11 µg/L) [11].To induce a PIP in the experiment, hospital strains of gram-positive cocci (Staphylococcus aureus, Staphylococcus epidermidis) and gram-negative bacteria (Escherichia coli, Pseudomonas aeruginosa) isolated from patients' pus were used on the skin of laboratory animals. The identification of the microorganisms was carried out at the educational-scientific bacteriological laboratory of the Department of Microbiology, Virology, and Immunology at Bukhara State Medical Institute, according to Bergy's Manual of Systematic Bacteriology [1997]. Nutrient media from the company "HiMedia" (India) were used for the bacteriological tests.The statistical processing of the obtained data was performed using the "Excel" program and methods of variational statistics. Statistical analysis was performed on a personal computer based on a "Pentium IV" processor using a software package for medical and biological research.
4. Results and Discussion
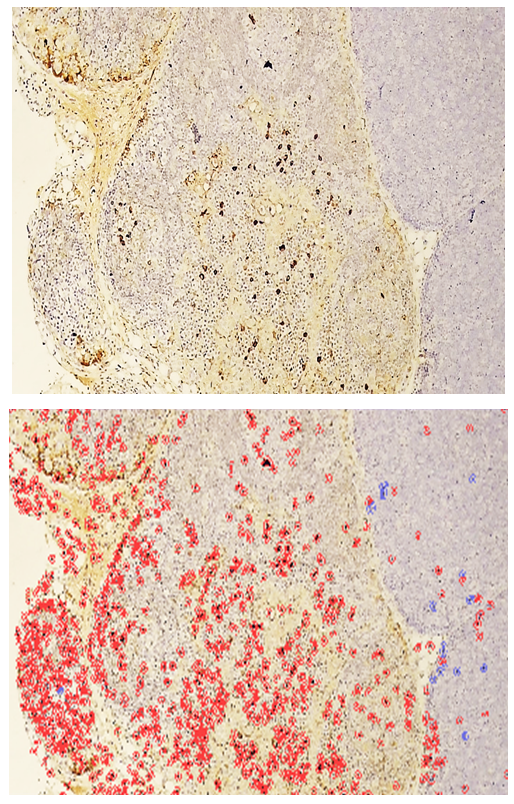
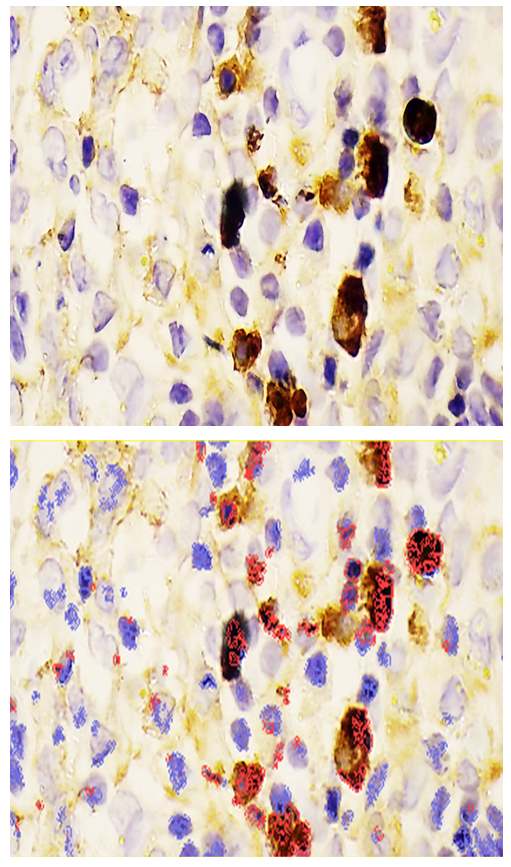
- Following euthanasia, thymi and lesional skin tissues were harvested from the outbred white rats used in the study. Histological slides were prepared from these tissues for an IHC study. For this purpose, the slides prepared from the thymus were stained using the DAB chromogen method, viewed at 400x magnification, and scanned with the QuPath-0.4.0.ink program. The resulting image is presented in the form of Figure 1.
|
|
|
|
5. Conclusions
- In the thymus tissues of laboratory animals with purulent-inflammatory diseases (PIDs) caused by gram-positive cocci (Staphylococcus aureus, Staphylococcus epidermidis), the CD-138 immunological marker of plasma cell membrane protein was highly expressed, with a positive expression rate of 98.50%. The experiment proved that the CD-138 marker was highly expressed in thymus tissues.The high level of CD-138 marker expression in the thymus tissues indicated the presence of an active PID in the animal's body with gram-positive cocci as the etiological agents. It was shown that the expression level of the CD-138 marker in thymus tissues using the IHC method has not only diagnostic but also prognostic significance.Unlike PIDs caused by gram-positive cocci, those caused by strains of gram-negative bacteria (Escherichia coli, Pseudomonas aeruginosa) showed a moderate level of CD-138 marker expression in thymus tissues. Correspondingly, the positive expression parameter decreased to 41.19%. It was proven that the expression level in PIDs caused by gram-negative bacteria is lower than in those caused by gram-positive cocci.The quantitative ratio of positive and negative cells and the positive expression indicators showed that the expression level of the CD-138 marker differed in PIDs caused by gram-positive cocci and gram-negative bacteria. High-level expression of the CD-138 marker was found with highly pathogenic causative agents, while moderate-level expression was found when the etiological agents had low pathogenicity.A high level of S-100 marker expression was found in skin tissues observed in the histological slide, with a positive expression of 94.75%. Similar to the thymus, the high expression of the S-100 marker in the skin was a characteristic of PIDs caused by gram-positive cocci.It was also proven that the S-100 marker was highly expressed in skin tissues in PIDs caused by gram-negative bacteria. The percentage of positive expression remained high, at 92.61%. This finding allows for the recommendation of using S-100 marker expression in skin tissues for both diagnostic and prognostic purposes, as this IHC method also provided the opportunity to evaluate the severity of PIDs in the skin based on the causative agents.
Conflict of Interest
- The authors declare no conflicts of interest.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML