Malikov Nodir Muzaffar ugli1, Ergashev Ulugbek Yusufjanovich2, Iriskulov Bakhtiyor Uktamovich3
1Assistant, Department of General Surgery No.2, Tashkent State Medical University, Tashkent, Uzbekistan
2DSc, Professor, Head of Department of General Surgery No.2, Tashkent State Medical University, Tashkent, Uzbekistan
3DSc, Professor, Head of Department of Normal and Pathological Physiology, Tashkent State Medical University, Tashkent, Uzbekistan
Correspondence to: Malikov Nodir Muzaffar ugli, Assistant, Department of General Surgery No.2, Tashkent State Medical University, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
At the same time, worldwide, diabetes mellitus remains a widespread, urgent disease with frequent comorbidities, frequent complications, and unresolved problems. Globally, currently, 537 million out of every 10 adults aged 20 to 79 are diagnosed with DM. According to the International Diabetic Federation, the incidence of DM among the adult population will reach 552 million people by 2030. The effectiveness and significance of bioplastic collagen preparations in the treatment of acute and chronic ulcers have been undeniably confirmed in the literature. Medical preparations based on collagen have already been introduced into practice, and the pathophysiological and surgical study of the effective stimulation of the regenerative and proliferative periods in the wound healing stage will give impetus to the experimental use of composite collagen in DFS. Based on this, an experimental study was conducted on the short-term healing of wound defects with the experimental use of composite collagen in DFS.
Keywords:
Bioplastic collagen, Composite collagen (collagen + quercetin), Experimental diabetic foot syndrome, Vascular endothelial growth factor, Cytokines, Insulin-like growth factors
Cite this paper: Malikov Nodir Muzaffar ugli, Ergashev Ulugbek Yusufjanovich, Iriskulov Bakhtiyor Uktamovich, Elimination of the Wound Defect in Experimental Diabetic Foot Syndrome Using Collagen in Composite Form, American Journal of Medicine and Medical Sciences, Vol. 15 No. 9, 2025, pp. 2875-2885. doi: 10.5923/j.ajmms.20251509.07.
1. Introduction
According to the International Diabetes Federation (IDF), 463 million people are currently diagnosed with diabetes, and by 2045, 700 million people will be diagnosed with it. In Central Asian countries, including Uzbekistan, as of 2015, 155,000 people were diagnosed with DM, in Kyrgyzstan - 46,917, and in Tajikistan - 30,000 [1,2].As of 2023, the population of the Republic of Uzbekistan was 367,998,00 people, of which 35.8% (12,893,600 people) were aged 31-59, 26.8% (96,544,00 people) were 14-30 years old, 28.3% were children under 14 years old, and 9.1% were over 60 years old. According to the 2023 statistical data of the Republican Scientific Center of Endocrinology, 37,7115 people with type II DM are registered with dispensaries, and 26,3526 people with one or another degree of obesity. In this case, for every 1024.77 people out of 100,000, type II DM is diagnosed, and 716.11 people suffer from obesity [3].In terms of mortality, DM ranks 3rd after cardiovascular and oncological diseases, with 300,000 people dying from this disease annually [4].According to the World Health Organization (WHO), this disease is a new epidemic of a non-infectious nature with a chronic degenerative course, threatening the lives of patients [5].The dominant trend in modern wound dressings (WB) is the use of composite polymer matrices based on natural polymers with biomast, biodegradation, and the addition of a bioactive additive [6]. Today, there are more than 300 types of JQ, the number of which has significantly increased in recent years [7,8,9,10].In the literature, it is noted that the bioplastic material "Collost" with sterile collagen has been successfully used in the treatment of DFS wounds. In this case, this material effectively studied the manifestation of granulation tissue growth and epithelialization stimulation in the local treatment of DFS complicated by purulent-necrotic wounds [11]. The bioplastic material collost represents type I collagen, which retains its nativity. When using collost, complete wound sanitation is not required, it is sufficient to clear the fibrin and necrotic tissue around the wound. The dressing can be changed every 2-3 days as the wound becomes moist. According to scientists, it is preferable to use a colostum in the 2nd-3rd stages of the wound course [12].The reason we are talking about flavonoids is that the quercetin substance in composite collagen belongs to this group. Flavonoids are widely used in medicine today due to their numerous pharmacological effects. Flavonoids are polyphenolic compounds that form two aromatic rings (A and B) through a three-carbon bridge of 15 carbon atoms. Quercetin, a flavonol, belongs to one of the six classes of flavonoids and is obtained from plants. The use of quercetin as an antioxidant is manifested in its ability to bind metal ions to form a chelate complex and inhibition of free radicals [13,14]. Quercetin contributes to the absorption of microthrombi by reducing platelet aggregation, enhances microcirculation, and strengthens blood vessel walls [15]. Quercetin reduces complications of diabetes mellitus caused by oxidative stress, including nephropathy and cognitive impairment. Due to its hepatoprotective antioxidant activity, it is used for liver damage [16]. Quercetin is cited in the literature as an anti-tumor agent, the body's fight against it through the apoptosis of defective cells [17]. In addition, quercetin is used as an immunomodulator, as well as an anti-allergic agent by participating in the stabilization of mast cell membranes by reducing histamine production [18].
2. Materials and Methods
For experimental research, 190 outbred white male rats weighing 190-250 grams, kept in the vivarium of the Tashkent Medical Academy, were taken. Rats were kept in optimal conditions. All operations and manipulations performed on animals were performed under general anesthesia, in full compliance with the humanitarian principles set forth in the guidelines of the European Community (86/609/EEC) and the "Rules for Working with Experimental Animals" of the Helsinki Declaration. The experimental group consisted of a total of 180 rats, in addition, 10 rats were taken for the intact group. After 24 hours of fasting, the animals were weighed and, accordingly, 2% alloxan, dissolved in 0.9% physiological saline, was administered intraperitoneally once at a dose of 12 mg/100 g of body weight. Thus, the experimental animals were divided into 4 groups: 1st group - unchanged group (intact); 2nd group (control group) - improvement of the experimental model of wound defect formation in the animal's foot against the background of alloxan diabetes; 3rd group (comparison group) - traditional complex treatment of a wound defect formed against the background of alloxan diabetes; Group 4 (main group) treatment with composite collagen. 60 rats were taken for each group, respectively. After surgery, the rats were divided into 3 groups depending on the treatment methods:1- control group (traditional wound cleansing, i.e., with a 3% solution of hydrogen peroxide);2- comparison group (applying levomecol to the wound in addition to traditional wound cleansing);3- a new local composite of collagen (collagen + quercetin) was applied to the wound of the main group once every other day after the traditional cleansing of the defect. (see Figures 1-a and b).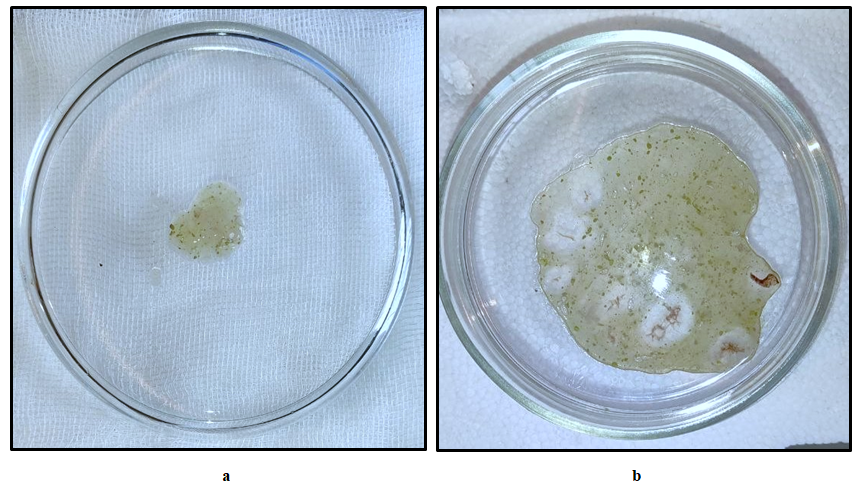 | Figure 1 a, b. Composite collagen prepared for wound application |
Dynamic observation was conducted every 1, 3, 7, 10, 14 days from the start of treatment. In all groups, no deaths were observed until the last day of the experiment (17 days). Within the specified period, the general condition of the animals was assessed, the area of the wound was measured, the affected area was photographed, and daily diuresis was determined. Within the specified period, the animals were decapitated, blood was taken for hematological and biochemical analysis, and the affected area (diabetic foot), liver lobule, and endocrine part of the pancreas were taken for morphological examination.Special verification methods. In order to determine wound healing indicators, vascular endothelial growth factor (VEGF-A164 - vascular endothelial growth factor) and insulin-like growth factor (insulin-like growth factor1, (IGF1) were determined using ELISA Kit for Vascular Endothelial Growth Factor A test system enzyme immunoassay analysis and "Right" analyzer of "Elabscience" (USA). Histological research methods. In this work, the morphological study was carried out on a carousel processor STP120 belonging to the German company Thermo-Fisher, after which the samples were placed in paraffin. Sections with a thickness of 3-4 μm were prepared on a rotary NM 325 microtome manufactured by TFS (USA). For Van Gieson staining of sections, serial sections were subjected to deparaffinization and dehydration. After washing the sections with distilled water, histological examination was performed using the direct microscope AxioLab A1 of Carl Zeiss (Germany). Statistical research methods. Statistical analysis of numerical data was carried out using the statistics program SPSS 16.0 and 6.0 for Windows. Mean values and standard deviations, medians and quartile intervals, as well as nonparametric methods for comparison (Mann-Whitney, Wilcoxon, Kraskelan-Wallis criteria) were determined. Statistical significance was taken into account when p<0.05 different.
3. Results
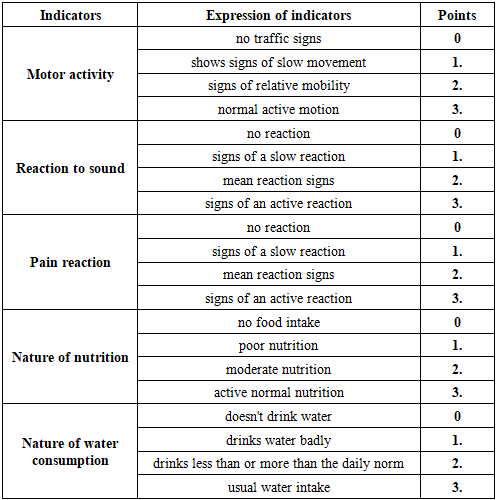
The general condition of the experimental animals was assessed based on the arithmetic mean of the scores obtained according to the following criteria. This included motor activity, reaction to sound and pain, the nature of nutrition and water intake (see Table 1).Table 1. Assessment of the general condition of laboratory animals with experimental diabetic foot
 |
| |
|
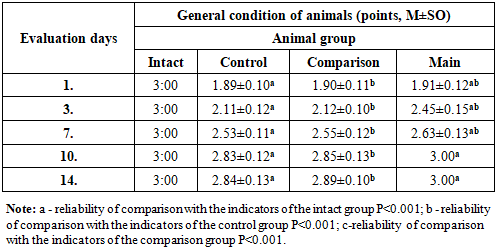
Table 2. Dynamic assessment of the general condition of laboratory animals with experimental diabetic foot
 |
| |
|
The integral score indicator of the general condition of rats with alloxan diabetes mellitus depended on the type of treatment and had a tendency to change in all groups during the experiment. During the treatment of the diabetic foot model, by the 3rd day of the experiment, the indicators of the integral assessment of their general condition in all groups of rats differed significantly (Table 2).Treatment of diabetic foot wounds (standard wound cleansing + levomekol) was practically equal to the indicators of the animals of the comparison group and the control group without treatment (P<0.001) and did not differ at all during the experiment. In this case, as can be seen from the integral scores for assessing the general condition of the animals, the treatment of the comparison group by the 14th day of the experiment was equal to the values of intact rats.We also observed that the indicators of the general condition of the animals of the main group in the first three days of the experiment were similar to the values of the control and comparison groups (P<0.001), and by the 10th and 14th days of the experiment, they were equal to the indicators of the general condition of intact rats.Thus, while the above-mentioned criteria for the integral assessment of the general condition of animals made it possible to determine their objective clinical condition, a significant positive change in this dynamics can be observed in relation to the main and comparison groups.The diabetes model led to a significant change in the main biochemical indicators. If we pay attention to Figure 2, then on the 1st day of the diabetic foot model, the blood glucose level in rats increased statistically by 2.87 times compared to the values of intact rats and amounted to 16.8±0.32 mmol/l (P<0.001) (in intact rats - 5.7±0.18 mmol/l). In the following days, we observed a slight trend towards a change in blood glucose levels in diabetic rats. However, the glucose level remained high and exceeded the values of intact rats by 2.67 times (P<0.001); On days 3 and 7, as well as on days 10-14, 2.45 (P<0.001); 2.39 (P<0.001) and 2.22 times (P<0.001), respectively 15.5±0.3; 14.3±0.22; 13.9±0.18 and 12.85±0.23 mmol/l. The obtained analyses indicate the long-term persistence of hyperglycemia in experimental animals and its adequacy to the model used. Of course, we didn't focus on reducing blood glucose levels. The effect of composite collagen on the wound defect in experimental animals against the background of diabetes mellitus was carried out precisely against the background of hyperglycemia, and insulin therapy or other additional types of glucose-reducing therapy were not used in humans, as in the case of wound complications of diabetes mellitus against the background of hyperglycemia. Nevertheless, a slight hypoglycemic effect of quercetin in composite collagen can be observed (see Figure 2).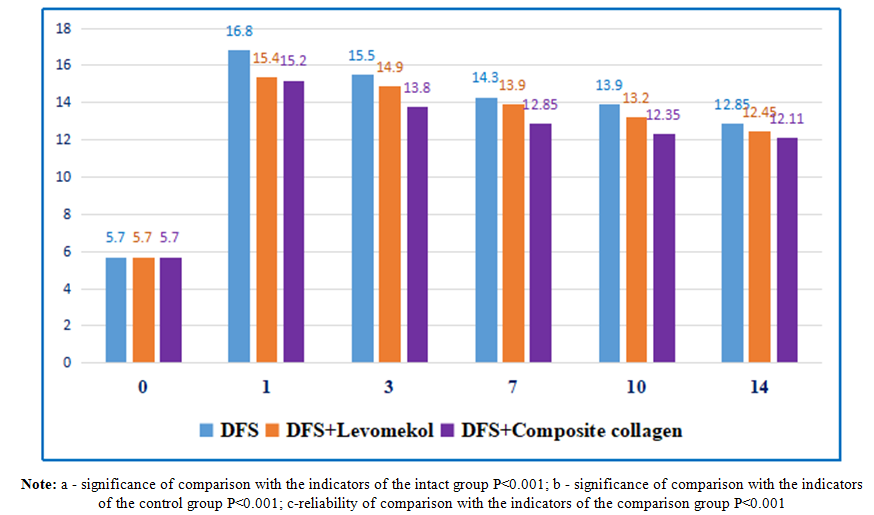 | Figure 2. Dynamic changes in the amount of glucose (mmol/l) in capillary blood of experimental diabetic rats |
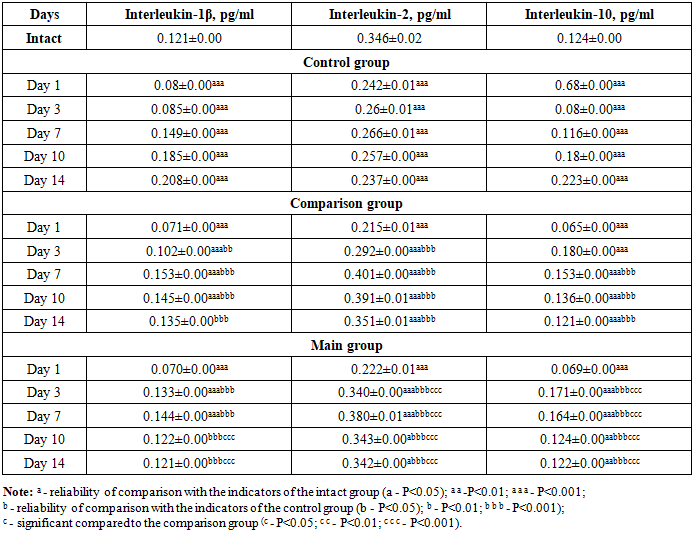
It should be noted that in our work we did not focus on reducing the level of glucose in the blood of rats, that is, we observed the state of diabetes. The glucose content in the rats of the comparison group was significantly higher than the values of the animals of the intact group, practically not differing from the control group on all days of the experiment. In general, the values of the main group did not differ as much as the values of the comparison group.We also studied the activity of cytokines responsible for the inflammation and healing of wound defects in the blood plasma of rats. Cytokines are produced by the synthesis of monocytes after their transformation into tissue macrophages at the site of inflammation. These cytokines consist of a combination of pro- and anti-inflammatory cytokines.Thus, by studying the complex of interleukins (interleukin-1β, interleukin-2, interleukin-10) from cytokines, we obtained the following results (see Table 3).Table 3. Dynamics of changes in interleukin indicators in blood plasma of experimental animals, M±m
 |
| |
|
In the comparison group, on the 1st day, the level of IL-1β, IL-2, IL-10 tended to increase slightly compared to the control group, and only IL-10 showed a tendency to decrease, i.e., equal to 10.39 times (P<0.01). We attributed this to the presence of immunomodulatory properties in the treatment transferred to the comparison group, as it blocks cytokine recognition receptors in the first days of inflammation. On the 3rd day, there was an increase in IL-1β by 1.25 times (P<0.001), which we explained by the fact that the treatment carried out in the comparison group stimulated the production of cells responsible for the immune system. We found a tendency to increase IL-2 by 1.18 times (P<0.001) compared to the control group, and we also observed an increase in the value of IL-10 by 2.1 times (P<0.001) by the same day. On the 7th day, a critical tendency was observed in all interleukins compared to the control group, increasing by 1.05 times (P<0.0001); 1.55 times (P<0.001) and 1.32 times (P<0.001). By the 10th day of the experiment, the effectiveness of the treatment method decreased in all studied interleukins (except IL-2) compared to the control group by 1.24 times (P<0.001); 1.35 times (P<0.001); In IL-2, an increase of 1.57 times (P<0.001) was observed. On the 14th day, it proceeded with an equal tendency compared to the intact group, and only in IL-2 remained at 0.354±0.00 pg/ml.When comparing the cytokines in the blood plasma of rats in the main group with the comparison group, on day 1, IL-1β remained practically unchanged, while in IL-2 there was a 1.01 times (P<0.001) higher trend, and in IL-10 there were practically no changes. This is due to the high immunomodulatory properties of composite collagen. On the 3rd day, IL-1β decreased by 1.30 times compared to the control group (P<0.001); In IL-2, there was a tendency to increase by 1.17 times (P<0.001). Since IL-10 was in the wound healing stage, there was a tendency to decrease by 1.044 times (p<0.001) compared to the comparison group. By day 7, we observed that inflammatory IL-1β reached the highest critical point, which was equal to 0.145±0.00 pg/ml. In IL-2 and IL-10, a decrease was observed compared to the comparison group, which was equal to 0.380±0.01 pg/ml and 0.164±0.00 pg/ml, respectively.The obtained analyses have proven that the use of composite collagen in wound defects of diabetic foot rats has a positive and desirable effect on immunomodulatory properties and wound healing activity compared to the comparison group (levomecol).VEGF-A164in the blood of experimental animals showed significant dynamic changes during the experiment (see Table 4). We did not consider it necessary to determine VEGF-A in the enzyme-linked immunosorbent assay of blood plasma of intact rats.Table 4. Dynamics of changes in VEGF-A (pg/ml) indicators in blood plasma of experimental animals, M±m
 |
| |
|
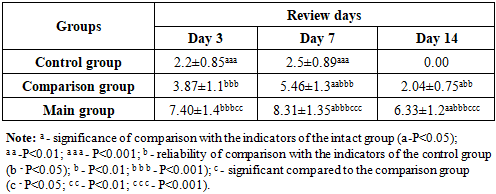
In the blood plasma of rats of the comparison group with diabetic claws, the level of VEGF-A on the 3rd day of the experiment increased by 1.76 times (P<0.001); On the 7th and 14th days, it was 2.18 (P<0.001) and 2.04 times (P<0.001) higher than the indicators of the control group rats, respectively. We attributed this to the fact that the ointment (levomecol) applied to the wound defect of animals in the comparison group had a positive effect on the reparative processes in the wound.It is known that VEGF-A, even against the background of ischemia in the body of animals, can manifest itself as an increase in its level in the blood as the main sign. An increase in its (VEGF) content in rats of the control group may have influenced not only compensatory mechanisms, but also the state of ischemia of the wound surface, which arose against the background of diabetes mellitus.The content of VEGF-A in the blood plasma of experimental animals of the main group with wound defects of the diabetic foot against the background of treatment with composite collagen on the 3rd day of the experiment was 1.91 (P<0.001) and 3.36 times (P<0.001) significantly higher than in the comparison and control groups, and on the 7th and 14th days - 1.52 (P<0.001) and 3.32 (P<0.001) times, respectively; 3.10 (P<0.001) and 6.33 (P<0.001) times higher (Fig. 3).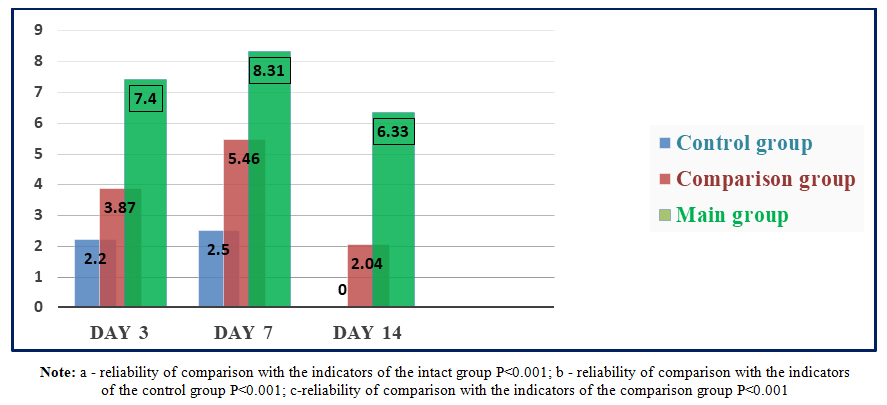 | Figure 3. Dynamics of VEGF-A levels (pg/ml) in the blood of animals of the main group with the use of composite collagen |
It can be seen that, both under normal conditions and in the blood of rats, IGF-1 is present on average in the amount of about 65 ng/ml (Table 5). However, against the background of alloxan diabetes, a slight decrease in this growth factor can be observed in the rats of the control group. We observed a significant increase in the content of IGF-1 in the blood plasma of animals of the comparison group by 1.246 times (P<0.001) on the 3rd day of the experiment compared to the values of rats of the control group. When conducting the same comparison on the 7th and 14th days of the experiment, it can be seen that the amount of IGF-1 in the plasma of the animals of the comparison group increased by 1.305 (P<0.001) and 1.282 (P<0.001) times, respectively. We attributed this to the manifestation of the regenerative properties of the levomecol ointment applied to the wound of diabetic paw animals of the comparison group (against the background of standard wound cleansing). Of course, the level of IGF-1 on the 14th day of the experiment in the animals of the comparison group was slightly lower than on the 7th day, which indicates its approach to normal values (intact) against the background of wound healing.Table 5. Dynamics of changes in IGF-1 (ng/ml) indicators in blood plasma of experimental animals, M±m
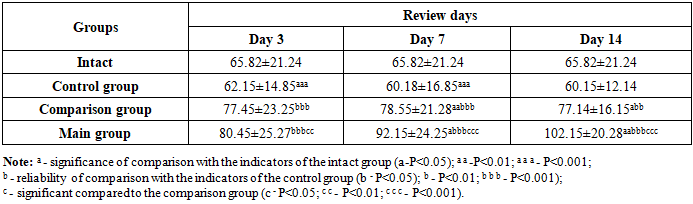 |
| |
|
Against the background of the use of composite collagen in rats with diabetic foot lesions (main group), we observed a tendency towards a significant increase in the content of IGF-1 in the plasma of animals on all days of the experiment (3, 7, 14) (see Fig. 4).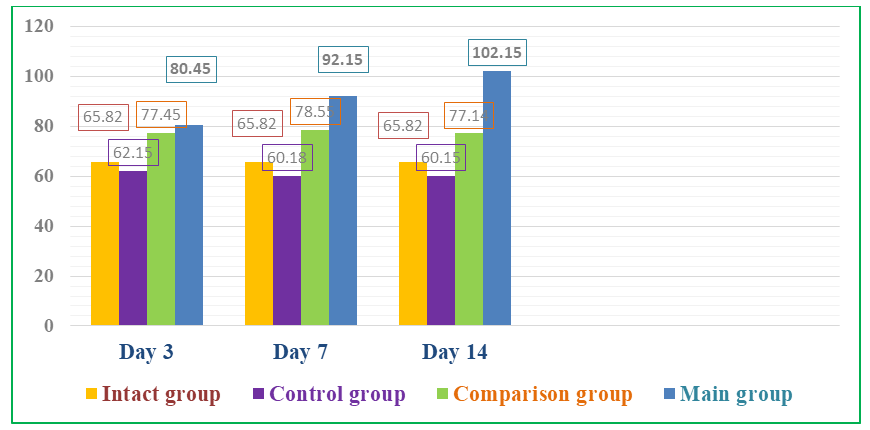 | Figure 4. Dynamics of the level of IGF-1 (ng/ml) in the blood of animals of the main group with the use of composite collagen |
The content of IGF-1 in the plasma of the main group of rats receiving composite collagen (collagen Itip + quercetin) on days 3, 7, and 14 of the experiment was 1.22 (P<0.001); 1.40 (P<0.001) and 1.55 (P<0.001) times, compared to the comparison group, on the 3rd day of the experiment by 1.038 (P<0.001) times, and on the 7th and 14th days of the experiment by 1.17 (P<0.001) and 1.32 (P<0.001) times, respectively. We attributed this to the fact that the collagen type in the composite collagen forms a matrix in the wound defect and involves the specific collagen and fibroblasts of the animal body in the wound healing process, an increase in the activity of tissue growth factors (in plasma) against the background of the enhancement of microcirculation by quercetin. The highest fluctuation of IGF-1 in the main group indicates a significant influence of composite collagen on wound healing, regeneration, and reparative processes.Studies have shown that in the control group of rats, an increase in the size of the wound was observed one day (24 hours) after the injury, which can be attributed to the fact that the edges of the wound diverged from each other due to edema, and the edema spread throughout the leg (Fig. 5 and 6).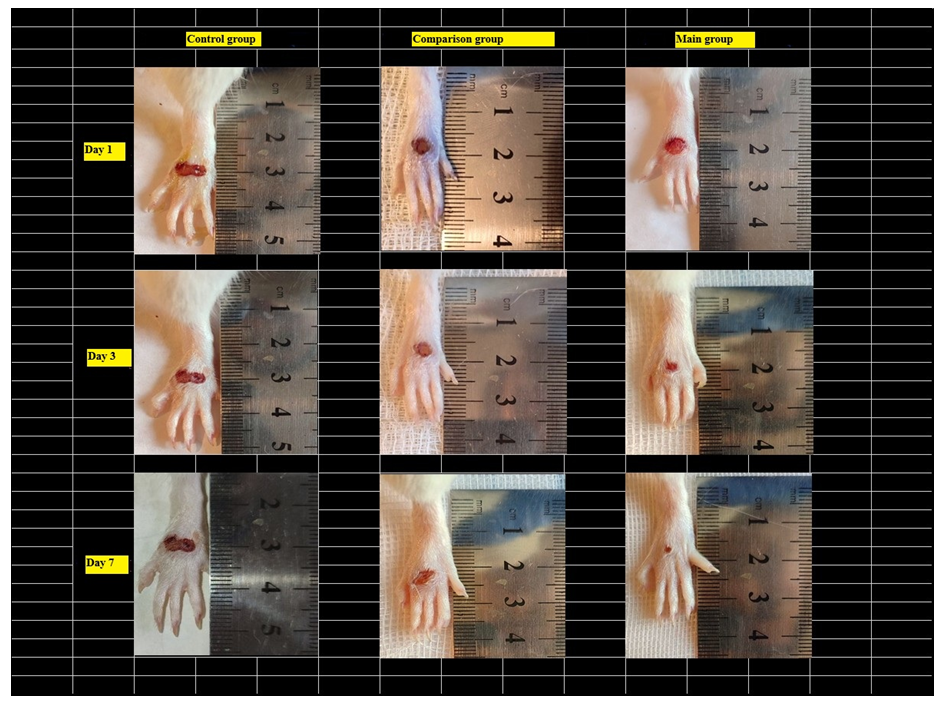 | Figure 5. Comparison of images of foot injuries and the size (area) of injuries on days 1, 3, and 7 after the occurrence of a diabetic wound defect between the control, comparison, and main groups |
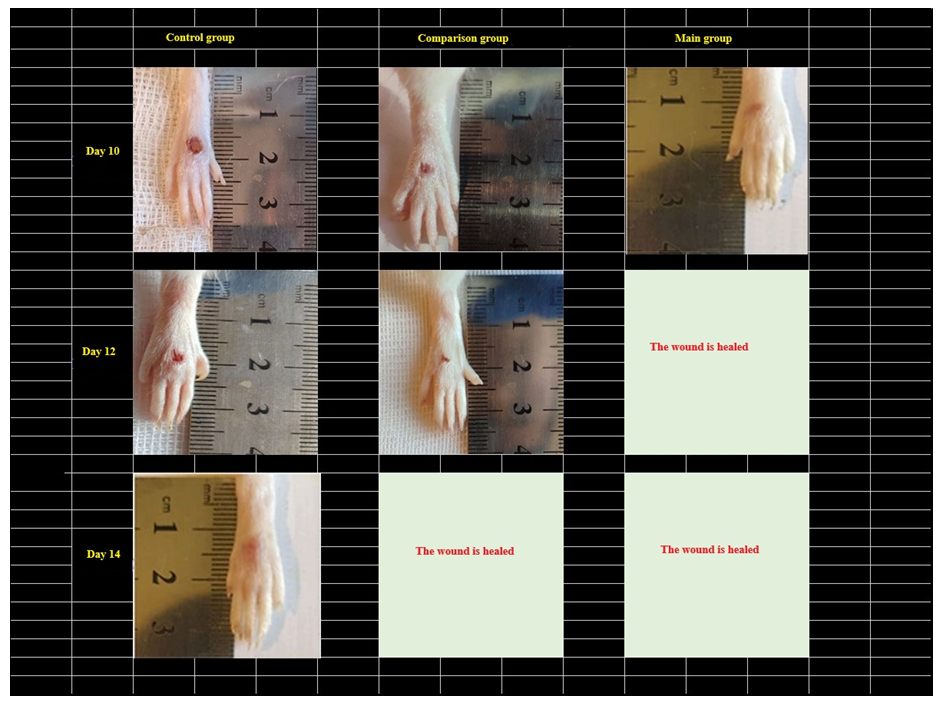 | Figure 6. Comparison of images of foot injuries and the size (area) of injuries on days 10, 12 and 14 after the occurrence of a diabetic wound defect between the control, comparison, and main groups |
On the 1st day of the wound model on the skin of the foot, the wound surface was covered with a thin scab due to discharge from it. When the animal was examined by hand, it was observed that the scabbard of the wound easily separated and serous fluid was released from its surroundings. Pronounced signs of inflammation were observed; edema of the wound edges, signs of necrosis and hyperemia, and the appearance of fibrin fibers at the base of the wound.On the 7th and 10th days of the experiment, the thinning of the epidermis in several places during morphological examination of the comparison group indicates that the wound healing process was slow or insufficient. In the dermis, one can see distinct structures of red-stained collagen fibers. However, the fact that collagen does not fully manifest its structures indicates that wound healing has not been completed. In a number of places, the collagen structure manifested as a soft-porous and poorly organized network, which indicates the continuation of the inflammatory process. Mild hyperemia of blood vessels is a typical sign of wound healing, indicating a slight increase in microcirculation. Hemostasis in several places is a sign of chronic inflammation. An inflammatory infiltrate consisting of macrophages and neutrophils can be seen in the dermis. This, in turn, requires cleansing the wound of necrotic tissue to reduce active inflammation and accelerate wound healing. The manifestation of granulation tissue is an important factor in wound healing. Despite a number of deficiencies in the structure of specific collagen, active wound healing continues (Fig. 7a, b).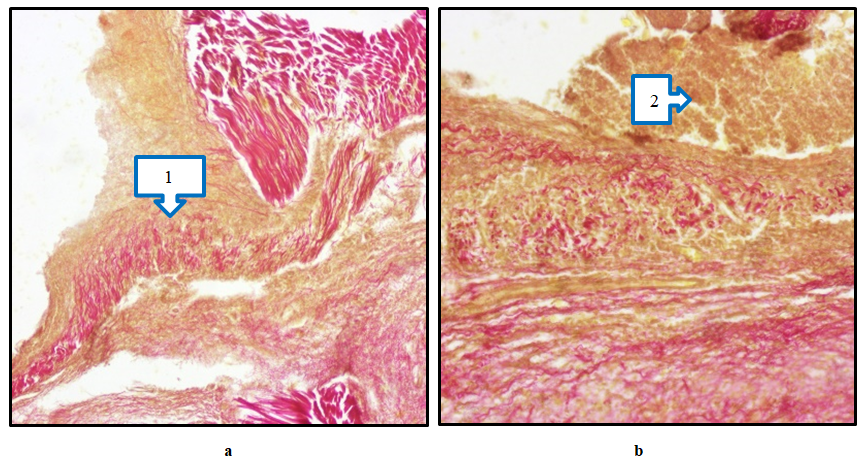 | Figure 7. Diabetic foot, Comparison group. Preservation of necrotic mass (1) and inflammatory infiltrate (a) at the base of the wound on day 7. Appearance of a dense inflammatory infiltrate (2) around the wound of the diabetic foot on the 10th day of the experiment (b). Paint; Van Gieson. 20×20 |
On the 7th and 10th days of the experiment, when morphologically examining the limbs of rats of the main group, where composite collagen was used, bounded and separated destructive-necrotic tissues were detected on the wound surface (Fig. 8a,b). In this case, we observed that the epidermis was restored by pronounced regeneration cells, which indicates a progressive wound healing. The relatively ordered arrangement of collagen fibers in the dermis indicates tissue remodeling. This indicates active healing of the wound due to the restoration of connective tissue. Collagen fibers stained red indicate their transformation, indicating tissue remodeling and strengthening of the dermal structure. However, despite this, in some places the fibers are not yet properly organized, which is associated with the continued healing of the wound. Granulation tissue was relatively pronounced, and the formation of cells and collagen fibers was observed. This is due to the effective stable healing of the wound. These changes indicate the effective role of composite collagen material in wound healing. Reduction of the inflammatory process during wound healing, restoration of cellular elements and collagen fibers and vessels in the tissue indicate normalization of the skin structure.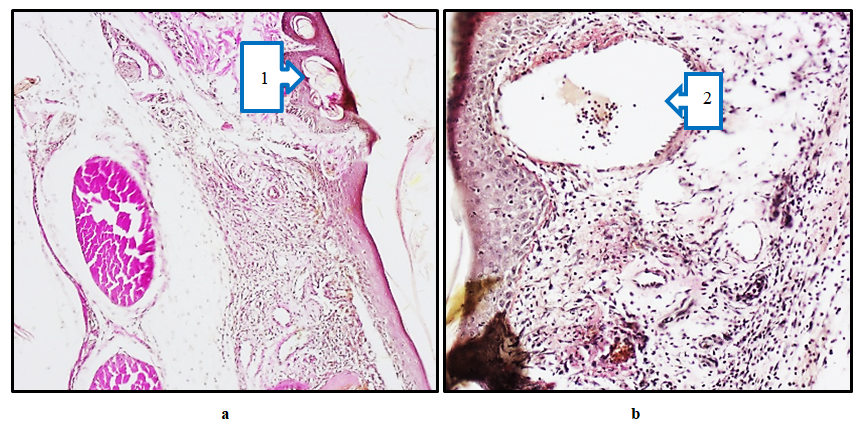 | Figure 8. Diabetic foot, Main group. Separation of necrotic tissue on the wound surface on day 7 (1) and remodeling of collagen fibers (a). On the 10th day of the experiment, densification of the epidermis surface layer (2) and fibrosis of connective tissue in the dermis (b). Paint; Van Gieson. 20×20 |
4. Discussions
We also studied the activity of cytokines responsible for the inflammation and healing of wound defects in the blood plasma of rats. When comparing the cytokines in the blood plasma of rats in the main group with the comparison group, on day 1, IL-1β remained practically unchanged, while in IL-2 there was a 1.01 times (P<0.001) higher trend, and in IL-10 there were practically no changes. This is due to the high immunomodulatory properties of composite collagen. On the 3rd day, IL-1β decreased by 1.30 times compared to the control group (P<0.001); In IL-2, there was a tendency to increase by 1.17 times (P<0.001). Since IL-10 was in the wound healing stage, there was a tendency to decrease by 1.044 times (p<0.001) compared to the comparison group. By day 7, we observed that inflammatory IL-1β reached the highest critical point, which was equal to 0.145±0.00 pg/ml. In IL-2 and IL-10, a decrease was observed compared to the comparison group, which was equal to 0.380±0.01 pg/ml and 0.164±0.00 pg/ml, respectively. The obtained analyses have proven that the use of composite collagen in wound defects of diabetic foot rats has a positive and desirable effect on immunomodulatory properties and wound healing activity compared to the comparison group (levomecol).Among the cytokines involved in the regulation of angiogenesis, the vascular endothelial growth factor plays an important role. Among the cytokines of this class (VEGF), the most studied peptide is VEGF-A. The value of VEGF-A in the blood plasma of rats of the control group was 2.2 pg/ml on the 3rd day of the experiment; On the 7th day, it remained practically unchanged and amounted to 2.5 pg/ml, significantly exceeding the values of intact rats (P<0.001). In the last days of the experiment, it did not differ from the indicators of intact rats. We attributed this to the fact that the compensatory mechanism in the animal body initially manifested itself in a slight increase in VEGF-A and, due to the absence of a stimulating factor, was equal to the values of intact rats. It is known that VEGF-A can manifest itself with an increase in its level in the blood as the main sign even against the background of ischemia in the body of animals. An increase in its (VEGF) content in rats of the control group may have been influenced not only by compensatory mechanisms, but also by the state of ischemia of the wound surface, arising against the background of diabetes mellitus. The content of VEGF-A in the blood plasma of experimental animals of the main group with wound defects of the diabetic foot against the background of treatment with composite collagen on the 3rd day of the experiment was 1.91 (P<0.001) and 3.36 times (P<0.001) significantly higher than in the comparison and control groups, and on the 7th and 14th days - 1.52 (P<0.001) and 3.32 (P<0.001) times, respectively; 3.10 (P<0.001) and 6.33 (P<0.001) times higher. Thus, against the background of the use of composite collagen, a sharp increase in the level of VEGF-A compared to the rest of the groups was expressed by the fact that the effect of the composite collagen used by us manifested itself in the influence of angiogenesis on the role of new tissue formation by stimulating regenerative and reparative processes of wound healing.Under normal conditions, IGF-1 is present in the blood of rats on average at around 65 ng/ml. However, against the background of alloxan diabetes, a slight decrease in this growth factor can be observed in rats of the control group. We observed that the content of IGF-1 in the blood plasma of animals of the control group on the 3rd day of the experiment decreased by 1.06 times (P<0.05) compared to the values of intact rats, and on the 7th and 14th days, the content of IGF-1 was almost 1.093 (P<0.001) and 1.094 (P<0.001) times, respectively, without significant differences. We attributed this to a decrease in insulin and a decrease in IGF-1 growth factor in the blood against the background of hyperglycemia without significant differences. The content of IGF-1 in the plasma of the main group of rats receiving composite collagen (collagen Itip + quercetin) on days 3, 7, and 14 of the experiment was 1.22 (P<0.001); 1.40 (P<0.001) and 1.55 (P<0.001) times, compared to the comparison group, on the 3rd day of the experiment by 1.038 (P<0.001) times, and on the 7th and 14th days of the experiment by 1.17 (P<0.001) and 1.32 (P<0.001) times, respectively. We attributed this to the fact that the collagen type in the composite collagen forms a matrix in the wound defect and involves specific collagen and fibroblasts in the wound healing process in the animal body, an increase in the activity of tissue growth factors (in plasma) against the background of an increase in microcirculation by quercetin. The highest level of IGF-1 fluctuation in the main group showed a significant influence of the composite collagen on wound healing, regeneration, and reparative processes.
5. Conclusions
1. When applying local composite collagen (collagen + quercetin) to the wound in the experimental diabetic foot (heel) model, it was observed that the cytokines in the blood plasma of the rats of the main group remained practically unchanged on the 1st day compared to the comparison group, while IL-1β had a tendency to increase by 1.01 times (P<0.001) compared to the control group, while IL-10 reached the highest critical point of 0.145±0.00 pg/ml on the middle days of the experiment, with practically no changes detected. In IL-2 and IL-10, a decrease was observed compared to the comparison group, which was equal to 0.380±0.01 pg/ml and 0.164±0.00 pg/ml, respectively, and the use of composite collagen in wound defects in diabetic rats showed a positive and desired effect on the immunomodulatory properties and wound healing activity compared to the treatment used in the comparison group.2. The use of composite collagen (collagen + quercetin) in the experimental diabetic foot model showed that the content of VEGF-A (cytokine) in the blood plasma of the main group of rats reached the highest critical point (7.40 and 8.31 pg/ml) on the 3rd and 7th days of the experiment compared to the indicators of the other groups of animals, which had a positive stimulating effect on the growth of new tissue against the background of enhancing the regulation of angiogenesis.3. In the experimental diabetic foot model, on days 7 and 14 of the experiment, the content of the insulin-like growth factor IGF (Insulin-like growth factors) in the blood plasma of the main group of rats was 92.15 and 105.15 ng/ml, respectively, which indicates a high reparative effect of composite collagen (collagen + quercetin) on tissue growth.4. As a result of treating the wound defect of the diabetic foot with composite collagen (collagen + quercetin), it was found that the acute exudative inflammation around the wound decreased earlier than in the wound of the remaining group of rats. In the dynamics of the conducted treatment, stabilization of dystrophic and destructive processes, activation of signs of reparative regeneration in the epidermis and dermis were observed, and on the 10th day, complete restoration of all cellular-fiber structures without signs of dystrophy and inflammation was revealed, and complete wound healing was noted (in the control group, the wound healed on the 14th day).
References
| [1] | Central Asian Diabetological Forum 2015 // Health of Kazakhstan Medical Newspaper. Almaty: 2015. - No. 3 (34). - P. 52-53. |
| [2] | International Diabetes Federation. IDF Diabetes Atlas, 9th edn. Brussels, Belgium: International Diabetes Federation. https://www.diabetesatlas.org/. |
| [3] | Kamalov T.T., Yunusova A.B. Dynamics of the prevalence of type 2 diabetes mellitus and obesity in Uzbekistan and Tashkent // Central Asian Endocrinological Journal. No. 1 /2024-104 p. |
| [4] | Artikova D.M., Shagazatova B.Kh., Urunbayeva D.A., Ishankulova N.F. Diabetic Foot Syndrome. Med jur // Bulletin of the Council of Young Scientists and Specialists of the Chelyabinsk Region No. 2 (9) 2015. P. 70-76. |
| [5] | Dedov I.I., Shestakova M.V., Andreeva E.N., et al. Diabetes mellitus: diagnosis, treatment, prevention; Edited by I.I. Dedov, M.V. Shestakova. - M., 2011. |
| [6] | Abidova A.D., Seomashko N.E., Aripova S.F. Obtaining components for wound coverings and assessment of their biological activity // Universe: Chemistry and Biology: electronic. scientific journal. 2019. No. 11 (65). URL: http://7universum.com/ru/nature/archive/item/7904. |
| [7] | Gladkova E.V., Norkin I.A., Belova S.V., Babushkina I.V., Mamonova I.A. Biodegradable wound cover and a method for obtaining biodegradable wound cover. Patent of the Russian Federation No 2519158, 2014. - 33 p. |
| [8] | Kirilenko Yu.K., Postnov S.E., Reshetov I.V., Chissov V.I., Yudanova T.N. Bandage for the treatment of wounds. Patent of the Russian Federation No. 2219954, 2003. |
| [9] | Kurinova M.A., Galbraikh L.S., Skibina D.E. Modern Wound Coverings (Review) // Modern Medicine: Current Issues: Collection of articles on the mother. XLVIII-XLIX international. scientific. - practical conf. - Novosibirsk: SibAK, 2015. No 10-11 (43). - P. 2-19. |
| [10] | Joshua S. Boateng Wound healing dressings and drug delivery systems: a review / Joshua S. Boateng [et al.] // Journal of Pharmaceutical Sciences. - 2008. - P. 97, - No. 8. - P. 282-293. |
| [11] | Stupin V.A., Goryunov S.V., Zhydkikh S.Yu., et al. Bioplastic collagen material collost in the treatment of patients with diabetic foot syndrome (results of a multicenter study). https://cyberleninka.ru/article/n/rol- kollagena-v-mehanizmah-zazhivleniya-hronicheskih-ran-pri-sindrome-diabetic-stopy/viewer. |
| [12] | Risman B.V. Treatment of Diabetic Foot Syndrome. Textbook for listeners of training of doctors, interns and the system of postgraduate training in the specialty "Surgery" / B. V. Risman - SPb.: "Onli-Press", 2016. - 76 p., ill. |
| [13] | Zverev Ya.F. Flavonoids as promising natural antioxidants // Bulletin of Medical Science. - 2017. - Vol. 1 - No5. - P. 20-27. |
| [14] | Tkachuk Z.U. [et al.] // Influence of the drug nuclex on the cytokine profile of patients with type 2 diabetes and the neuropathic form of diabetic foot // Int J Diabetes Res. - 2013. - Vol. 2, N 2. - P. 21-26. |
| [15] | Omonturdiyev S.Z. Study of the influence of quercetin flavonoid on the endothelium of the aortic crisis / S.Z. Omonturdiyev, T.D.U.L. Kadirov, D.R. Inomjonov, A.A.U.L. Abdullayev, B.J. Komilov, U.B.G Gaibov, T.F. Aripov // Universe: Chemistry and Biology. - 2021. - Vol. - No107. - P. 5-9. doi:10.32743/UniChem.2023.107.5.15403. |
| [16] | Susanne A. S. Safety Aspects of the Use of Quercetin as a Diet Supplement / A. S. Susanne, S. Pevny, R. Ziegenhagen, N. Bakhiya, B. Schäfer, K. Ildico Hirsch-Ernst, A. Lampen // Molecular Nutrition Food Research. - 2018. - Vol. - No. 1. - P. 18-23. DOI: 10.1002/mnfr.201700447. |
| [17] | Omar A.A. Molecular and biochemical investigations on the effect of quercetin on oxidative stress induced by cisplatin in rat kidneys // Saudi Journal of Biological Sciences - 2015. - No. 22. - P. 227-231. DOI: 10.1016/j.sjbs.2014.12.008. |
| [18] | Parhi B. Application of quercetin flavonoid-based hybrid nanocomposites: A review / B. Parhi, D. Bharatiya, S. K. Swain // Saudi Pharmaceutical Journal. - 2020. - Vol. 12. - No. 28. - P. d1719-1732. DOI.org/10.1016/j.jsps. |








 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML





