-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(9): 2860-2863
doi:10.5923/j.ajmms.20251509.04
Received: Aug. 11, 2025; Accepted: Aug. 28, 2025; Published: Sep. 2, 2025

Immunopathogenic Aspects of Predicting the Outcomes of Complex Treatment of Cancer of the Oral Mucosa
Ergashev Nodir Rustamovich
Republic Specialization Oncology and Radiology Applied Medical Science Bukhara Center Branch, Bukhara, Uzbekistan
Correspondence to: Ergashev Nodir Rustamovich, Republic Specialization Oncology and Radiology Applied Medical Science Bukhara Center Branch, Bukhara, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Oncological diseases of the oral mucosa include malignant neoplasms of the lips, tongue, floor of the mouth, cheek, gingival, retromolar and palatal areas, as well as the soft and hard palate. Oral mucosa cancer (OMC) remains a significant oncological challenge due to its aggressive behavior and frequent late diagnosis. The tumor microenvironment (TME) and host immune responses play crucial roles in disease progression and therapeutic outcomes. This review explores the immunopathogenic mechanisms influencing OMC, focusing on immune evasion, inflammatory mediators, and immune cell dysregulation. We also discuss predictive biomarkers that may enhance the precision of complex treatment strategies, including surgery, radiotherapy, chemotherapy, and immunotherapy. Understanding these immunopathogenic aspects could improve prognostic accuracy and guide personalized therapeutic approaches.
Keywords: RSOPR, TNM, CD, CD3+, CD4+
Cite this paper: Ergashev Nodir Rustamovich, Immunopathogenic Aspects of Predicting the Outcomes of Complex Treatment of Cancer of the Oral Mucosa, American Journal of Medicine and Medical Sciences, Vol. 15 No. 9, 2025, pp. 2860-2863. doi: 10.5923/j.ajmms.20251509.04.
Article Outline
1. Introduction
- According to the International Classification of Diseases (ICD-10), they are coded in the range C00-C06 and are classified as malignant tumors of the oral cavity and oropharynx. In the vast majority of cases, we are talking about squamous cell carcinoma, which accounts for up to 90-95% of all tumors in this localization [1].According to international statistics, oral cancer is one of the ten most common oncological diseases in men, ranking 6th to 8th in frequency among all malignant tumors, and more than 16th in women [2,3]. Despite its lower overall prevalence compared to lung, stomach, or breast tumors, oral oncology occupies an important place in oncological practice due to its high morbidity, tendency to local aggression, and significant impact on patients’ quality of life.According to GLOBOCAN, more than 377,000 new cases of oral cancer are registered annually in the world, and more than 177,000 patients die from this disease [4]. The incidence varies significantly depending on the region and level of economic development: the highest rates are registered in South and Southeast Asia (India, Sri Lanka, Pakistan), where the prevalence can reach 10-12 cases per 100,000 population, which is associated with the traditional use of betel, tobacco and alcohol [5]. In high-income countries (USA, Canada, Western European countries), stabilization or a moderate decrease in rates is noted, while in countries with transition economies and in developing regions of Central Asia, including the CIS countries, the incidence continues to grow. In particular, according to the oncology registries of the Republic of Uzbekistan, about 1,300 new cases of malignant tumors of the oral cavity are detected annually, with a tendency for the contingent of patients to become younger [6].Oral cancers not only carry a high risk of death, but also cause significant loss of quality of life, long-term disability, and severe economic consequences for the patient, family, and health care system. According to WHO estimates, the direct cost of treating head and neck cancer (including OC) in developed countries exceeds US$100,000 per patient per year, especially when combined therapy and reconstructive interventions are used [7]. Indirect costs (including loss of productivity and need for care) double this amount, especially in countries with poor social protection systems. According to an analysis by The Global Economic Cost of Cancer (American Cancer According to the Cancer Society, the total global cost of cancer, including oral cancer, was more than US$1.16 trillion in 2010, with up to 40% of the costs occurring in emerging economies [8].
2. Purpose of the Research
- The primary objective of this study is to elucidate the immunopathogenic mechanisms underlying oral mucosa cancer (OMC) and their influence on the outcomes of complex treatment modalities, including surgery, radiotherapy, chemotherapy, and immunotherapy. By analyzing the tumor microenvironment (TME), immune evasion strategies, and host inflammatory responses, this research aims to investigate the roles of immune checkpoint molecules (PD-1/PD-L1, CTLA-4), tumor-infiltrating lymphocytes (TILs), and inflammatory cytokines (IL-6, TNF-α, TGF-β) in OMC progression and treatment resistance, evaluate potential immunological and molecular markers that can forecast treatment response, recurrence risk, and overall survival, enabling personalized therapeutic strategies, assess how immunotherapeutic approaches and develop a more precise prognostic framework by incorporating immunopathogenic data, facilitating early intervention and tailored therapeutic regimens.Ultimately, this research seeks to bridge the gap between immunological insights and clinical applications, paving the way for more effective and individualized management of oral mucosa cancer.
3. Materials and Methods
- The work used clinical, morphological and immunological methods of assessment, including the study of cellular, humoral immunity, cytokines and inflammatory markers. For stratification of patients, both traditional prognostic scales and the author's immunological scale "ONIX" were used. Statistical analysis included Student's t-test, Mann-Whitney U-test, Pearson's χ² - test, correlation analysis, ROC-analysis and multifactorial logistic regression.
|
4. Results and Discussion
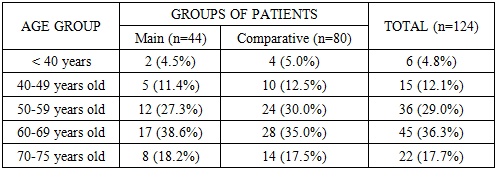
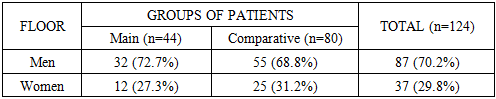
- The average age of patients in the main group was 63.1±6.9 years, and in the comparative group - 61.5±7.2 years, while the average age for the entire sample was 62.1±7.1 years. As can be seen from these average static values, the study sample is representative in terms of age composition for the RSOPR clinic and can serve as a reliable basis for analyzing prognostic factors and evaluating the results of complex treatment.In both groups, both the main group (patients with relapse) and the comparative group (patients with no signs of relapse during the 3-year follow-up), a predominance of men was noted. In the main group, men accounted for 72.7% of the total number of patients, and women - 27.3%. In the comparative group, the proportion of men was also leading (68.8%), women accounted for 31.2%. In general, in the aggregate sample, the proportion of men was 70.2%, which is more than twice the number of women (Table 2).
|
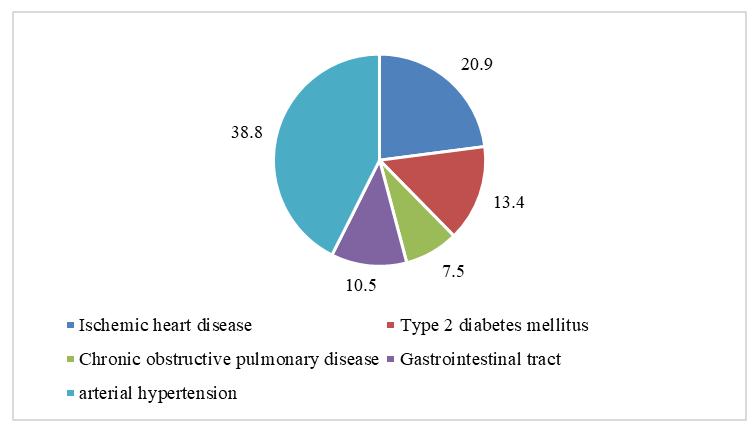 | Figure 1. Nature of comorbidities in the total sample of patients (n=124) |
5. Conclusions
- 1. Comparison of four traditional prognostic methods showed that the TNM classification had the best results (relapse prognosis - 41.9%, fact - 35.5%, AUC = 0.74), but it also demonstrated discrepancies in subgroups. The degree of differentiation (AUC = 0.70) gave apparently accurate final values, but was significantly mistaken for G1 and G3. ECOG (AUC = 0.68) underestimated the risk in patients with functional limitations. The least accurate was the clinical response to treatment (AUC = 0.66), in which the prognosis and fact differed by 3.9 percentage points.2. The immunological study showed that in patients with relapse of the disease, compared with both the group with a favorable clinical outcome and the reference values of healthy individuals, the indices of T-cell and NK-cell immunity (CD3⁺, CD4⁺, CD16⁺CD56⁺) were significantly reduced, the levels of proinflammatory and immunosuppressive cytokines (IL-6, IL-10) were increased, an increase in the level of circulating immune complexes (CIC) was detected, and signs of systemic inflammation (increased CRP , NLR, SII) were expressed . The revealed changes demonstrate a pathogenetic association with the risk of relapse and can be considered as an immunological basis for further development of a prognostic model.3. The developed method for predicting the outcome of complex treatment of RSOPR is based on an integrated assessment of immunological changes reflecting the depth of the antitumor control disorder. The proposed algorithm takes into account nine key indicators covering cellular immunity, cytokine profile and systemic inflammation. The obtained values are converted into a point scale allowing with high reproducibility to classify the patient into one of three categories: from favorable (with preserved immune surveillance and minimal signs of inflammation) to unfavorable (with pronounced immunosuppression, activation of proinflammatory cascades and loss of regulatory balance).4. Inclusion of immunological prognosis in traditional outcome assessment methods significantly increases the accuracy of relapse prediction after complex treatment. Thus, when using the TNM classification, sensitivity increased from 69.2% to 84.6%, and specificity - from 75% to 83.3%. For the ECOG scale, a similar increase was: in sensitivity - from 65.4% to 80.8%, in specificity - from 72.2% to 80.6%. When assessing the clinical response, the indicators improved from 61.5% to 76.9% and from 69.4% to 80.6%, respectively. Even when using the degree of histological differentiation, the addition of immunological parameters made it possible to increase sensitivity to 69.2% and specificity to 77.8%.
Conflict of Interest
- The authors declare no conflicts of interest.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML