Yarmatova Sh. Z.1, Navruzova N. O.2
1Independent Researcher, Department of Obstetrics and Gynecology, Bukhara State Medical Institute named after Abu Ali ibn Sino, Uzbekistan
2PhD, Assistant of the Department of Obstetrics and Gynecology, Bukhara State Medical Institute named after Abu Ali ibn Sino, Uzbekistan
Correspondence to: Yarmatova Sh. Z., Independent Researcher, Department of Obstetrics and Gynecology, Bukhara State Medical Institute named after Abu Ali ibn Sino, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Objective: To evaluate clinical, anamnestic, laboratory, and instrumental indicators in women with a risk of miscarriage. Materials and Methods: This study included 117 women in their first trimester of pregnancy from Bukhara and Karshi, all experiencing a threat of miscarriage. The participants were divided into three groups: Group 1 — 51 women with a risk of miscarriage after in vitro fertilization (IVF); Group 2 — 46 women with pregnancies up to 14 weeks after unsuccessful IVF attempts; Group 3 (control group) — 20 women with physiologically normal pregnancies. Results: The average age of patients was:Group 1: 23.61±0.46 years; Group 2: 28.17±0.8 years; Group 3: 28.25±0.75 years. Among the 90 patients in the main group, the most common cause of infertility was the tubal factor (39 women, 43.3%). Anovulationwas diagnosed in 31 women (34.4%), and idiopathic infertility in 20 women (22.2%). These findings emphasize the importance of a comprehensive and multidisciplinary approach to diagnosing and treating infertility. Even with physiologically progressing pregnancies, there is a risk of bleeding and thrombosis, which is associated with impaired fibrinolytic activity, hypercoagulation, endothelial dysfunction, and changes in blood rheology. When combined with thrombophilia, these factors often lead to complications in IVF pregnancies. Conclusion: Identified changes in the hemostasis system in the main group indicate a hypercoagulable state. This requires careful monitoring during IVF procedures, as a high risk of thrombosis can complicate treatment and reduce its effectiveness.
Keywords:
In vitro fertilization, Miscarriage, Ultrasound, Hemostasis, Hypercoagulation
Cite this paper: Yarmatova Sh. Z., Navruzova N. O., Clinical, Anamnestic, Laboratory, and Instrumental Parameters in Women with Threatened Miscarriage After IVF, American Journal of Medicine and Medical Sciences, Vol. 15 No. 9, 2025, pp. 2855-2859. doi: 10.5923/j.ajmms.20251509.03.
1. Introduction
Miscarriage, one of the obstetric complications occurring during pregnancy, remains a pressing medical issue. According to the World Health Organization, one out of every 4–5 pregnancies ends in spontaneous miscarriage, with 15–25% of these cases occurring during the first trimester [1].In particular, women who become pregnant through in vitro fertilization (IVF) have been shown to have a higher risk of miscarriage. This is often associated with conditions such as thrombophilia, hypercoagulation, and endothelial dysfunction [2,3]. Several studies have demonstrated that changes in the hemostatic system in IVF pregnancies — especially reduced fibrinolytic activity and increased tendency toward thrombosis — contribute to pregnancy loss [4,12].Thrombophilia is a group of inherited or acquired conditions that increase the risk of thrombus formation in the body. It can negatively affect pregnancy by disrupting placental blood circulation, thereby impairing fetal development [5,13]. Some studies have reported that in women with thrombophilia, the failure rate of in vitro fertilization (IVF) can increase by as much as 30–40% [6,11].Additionally, the hypercoagulable state observed during pregnancy is considered a natural protective mechanism of the body. However, if this condition becomes excessive, it can disrupt placental blood circulation, potentially leading to fetal retention failure or impaired fetal development [7,14].Clinical and laboratory studies indicate that in cases associated with miscarriage, there are notable changes in indicators such as D-dimer, fibrinogen, and the prothrombin index (PTI). Monitoring these parameters is critically important for reducing the risk of potential complications [8,9].The causes of infertility — including tubal factors, anovulation, and idiopathic conditions — are among the key factors influencing the success of pregnancy. Accurate diagnosis and appropriate treatment of these conditions significantly increase the chances of maintaining a viable pregnancy [10].
2. Objective
To evaluate the clinical, anamnestic, laboratory, and instrumental parameters in women with a history of miscarriage.
3. Materials and Methods
The study included 117 pregnant women in their first trimester from the cities of Bukhara and Karshi, all presenting with a threat of miscarriage. Participants were divided into three groups:Group 1 (n = 51): Women who underwent in vitro fertilization (IVF) and were at risk of miscarriageGroup 2 (n = 46): Women who became pregnant (up to 14 weeks’ gestation) following unsuccessful IVF attemptsGroup 3 (n = 20, control group): Women with physiologically normal pregnancies
4. Results and Discussion
The average age of all participants was as follows:• Group I: 23.61 ± 0.46 years• Group II: 28.17 ± 0.80 years• Control group: 28.25 ± 0.75 yearsA detailed age distribution of the examined women is presented in Table 1.Table 1
 |
| |
|
During the collection of anamnesis, we also considered the patients' place of residence. An analysis of the distribution by living area showed that in Groups I and II, the proportion of women from urban areas was approximately two times lower than that from rural areas — accounting for 33.33% and 36.96%, respectively.Table 2
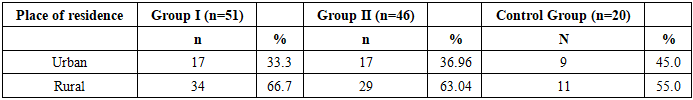 |
| |
|
An analysis of menstrual function in the study groups revealed that the age of menarche in the main groups occurred at age 12 in 11.8% of women, at age 13 in 29.4%, at age 14 in 41.2%, and at age 15 in 17.6% of cases (see Table 3). Late onset of menstruation was most commonly observed in women with endocrine infertility.Table 3. Age of menarche in women from the study groups
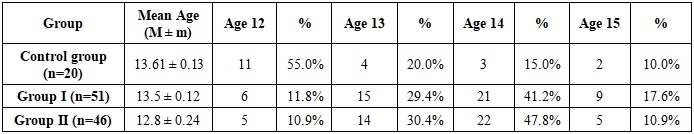 |
| |
|
Table 4 presents a detailed comparative description of the menstrual function in the examined women.Table 4. Comparative analysis of menstrual parameters in the study groups
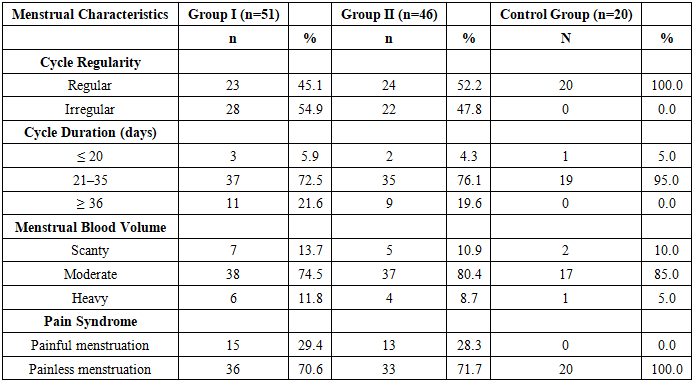 |
| |
|
In the studied groups, the age at the onset of sexual activity ranged from 18 to 25 years. Analysis of the average age at first sexual intercourse revealed the following: 20.1 ± 0.23 years in Group I, 21.2 ± 0.3 years in Group II, and 20.2 ± 0.34 years in the control group. No statistically significant differences were observed between the groups (p > 0.05) (see Table 5).Table 5. Average age at onset of sexual activity in study groups
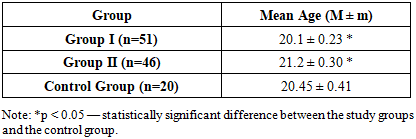 |
| |
|
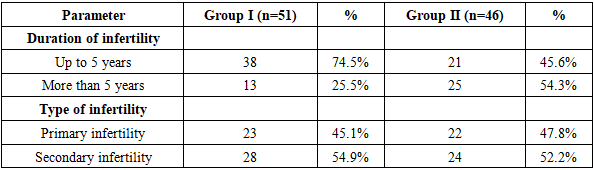
Considering the evidence from the literature regarding the impact of body mass index (BMI) on pregnancy, this parameter was also assessed in our study. In Group I, 9.8% of women had a BMI below 18.5, 41.2% had a BMI between 18.6 and 24.9, 37.3% were in the range of 25 to 29.9, and 7.8% had a BMI between 30 and 34.9. In Group II, the respective distributions were 8.7%, 43.5%, 34.8%, and 6.5%, while in the control group, the proportions were 5%, 85%, 10%, and 0%, respectively.According to the data from Table 6, the duration of infertility among the examined women was distributed as follows:In Group I, 38 women experienced infertility for up to 5 years, while the remaining 13 women had infertility lasting more than 5 years.In Group II, 21 women had infertility for up to 5 years, and 25 women for more than 5 years.Regarding the type of infertility:• In Group I, primary infertility was observed in 23 women, while secondary infertility was found in 28 women (54.9%).• In Group II, primary infertility was present in 22 women, and secondary infertility in 26 women.Table 6. Distribution of infertility duration among patients in the main groups
 |
| |
|
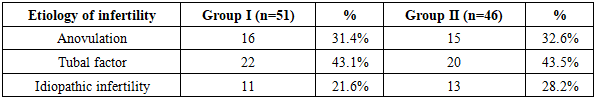
The etiology of infertility was categorized into three main types: anovulation, tubal factor, and idiopathic infertility. The corresponding results are presented in Table 7.Table 7. Distribution of patients by infertility factors
 |
| |
|
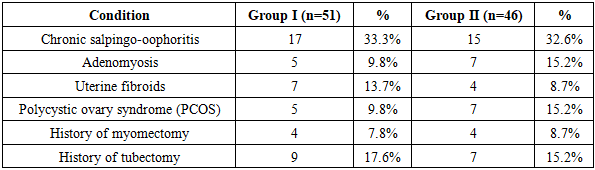
The study results indicate that among women who underwent in vitro fertilization (IVF) or became pregnant following unsuccessful IVF attempts, the tubal factor was the most prevalent cause of infertility (43.1% and 43.5%, respectively). Anovulation was also a widespread factor, observed at nearly equal rates in both groups (31.4% and 32.6%). The incidence of idiopathic infertility was higher in Group II (28.2%) compared to Group I (21.6%). These findings confirm that different clinical forms of infertility are critical factors influencing the success of IVF and the maintenance of pregnancy. Therefore, adopting an individualized approach, with treatment and monitoring strategies tailored to the specific etiology, is of great importance.In addition, special attention was given to the analysis of extragenital pathology, as such conditions may significantly impact women’s reproductive health and have various effects on fertility and overall pregnancy outcomes.Analysis of gynecological pathologies revealed that the most frequently observed condition in both groups was chronic salpingo-oophoritis, reported in 33.3% of cases in Group I and 32.6% in Group II. This finding suggests that it may play a significant role in the development of tubal factor infertility. Additionally, adenomyosis and polycystic ovary syndrome (PCOS) were more prevalent in Group II, indicating their potential negative impact on pregnancy progression. Uterine fibroids, as well as a history of myomectomy and tubectomy, are surgical factors that may also increase the risk of pregnancy loss (see Table 8).Table 8. Incidence of gynecological disorders in study groups
 |
| |
|
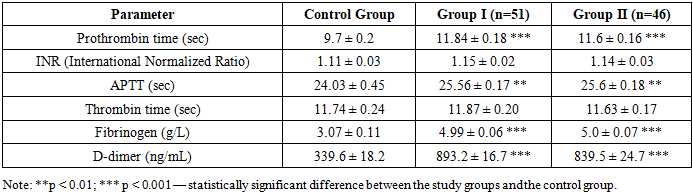
Table 9. Comparative description of blood coagulation parameters in the study groups (M ± m)
 |
| |
|
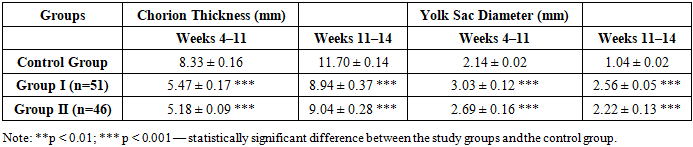
According to the study results, significant differences in blood coagulation parameters were observed in the study groups compared to the control group. In particular, the elevated levels of prothrombin time (8.6 ± 0.1 sec and 9.2 ± 0.11 sec), fibrinogen (4.97 ± 0.06 g/L and 5.02 ± 0.07 g/L), and a marked increase in D-dimer levels (893.2 ± 16.7 ng/mL and 839.5 ± 24.7 ng/mL) indicate enhanced coagulation activity and a tendency toward thrombotic states.Shortening of the APTT (23.3 ± 0.15 sec and 23.42 ± 0.16 sec) suggests activation of the intrinsic coagulation pathway, while a reduction in INR values (1.01 ± 0.02 and 1.02 ± 0.02) confirms a predisposition to hypercoagulability. These statistically significant differences support the presence of a hypercoagulable state in patients from the main study groups. All differences were considered statistically significant, with levels of p < 0.01 and p < 0.001.Table 10. Placental measurements in women with a risk of miscarriage (M ± m)
 |
| |
|
Ultrasound-based assessment of chorion thickness and yolk sac diameter revealed statistically significant differences between the groups. During weeks 4–11, chorion thickness was significantly lower in Group I (5.47 mm) and Group II (5.18 mm) compared to the control group (8.33 mm) (p < 0.001). These differences persisted through weeks 11–14. In contrast, yolk sac size was significantly larger in the main groups: Group I – 3.03 mm, Group II – 2.69 mm, while in the control group it was 2.14 mm during weeks 4–11 (p < 0.001). An enlarged yolk sac may be considered a biomarker indicating risk to normal fetal development. Such deviations are suggestive of delayed or pathological embryonic growth.
5. Conclusions
Thus, the identified clinical-anamnestic, laboratory, and instrumental changes in the studied patients highlight the multifactorial and complex nature of pregnancy maintenance problems. In particular, among women who underwent IVF, early evaluation of infertility causes, hemostasis abnormalities, and placental parameters is crucial for detecting pregnancy risk factors and implementing effective preventive strategies.
References
| [1] | WHO. Miscarriage: Facts and Statistics. World Health Organization. 2022. |
| [2] | Kutteh WH. Immunologic aspects of recurrent pregnancy loss. Clinical Obstetrics and Gynecology. 2015; 58(3): 517-529. |
| [3] | Carp H. Recurrent pregnancy loss: causes, controversies, and treatment. Taylor & Francis, 2014. |
| [4] | Brenner B. Haemostatic changes in pregnancy. Thrombosis Research. 2014; 114(5-6): 409-414. |
| [5] | Robertson L, Wu O, Langhorne P, et al. Thrombophilia in pregnancy: a systematic review. BJOG. 2016; 113(5): 560–570. |
| [6] | Martinelli I, et al. Thrombophilia and pregnancy complications. Haematologica. 2013; 88(6): 624-629. |
| [7] | Hellgren M. Hemostasis during normal pregnancy and puerperium. Seminars in Thrombosis and Hemostasis. 2023; 29(2): 125-130. |
| [8] | Di Nisio M, et al. D-dimer test in pregnancy: a systematic review. Journal of Thrombosis and Haemostasis. 2017; 5(5): 1113–1118. |
| [9] | Clark P, Brennand J. Antenatal screening for thrombophilia in pregnancy. Current Opinion in Obstetrics and Gynecology. 2009; 21(2): 128-133. |
| [10] | Practice Committee of the American Society for Reproductive Medicine. Evaluation and treatment of recurrent pregnancy loss. Fertility and Sterility. 2012; 98(5): 1103-1111. |
| [11] | Ixtiyarova, G., Sharipova, D., Kilicheva, V., & Xafizova, D. (2018). THE PROBLEM OF ANTENATAL POTHOLOGY AND THE WAYS OF ITS SOTUTION. BRIDGE TO SCIENCE: RESEARCH WORKS, 203. |
| [12] | Ikhtiyarova, G. A., Khafizova, D. B., & Ikhtiyarova, D. F. (2019). Criteria For prediction of complications in pregnant women with antenatal fetal death. ТОМ VI, 67. |
| [13] | Ikhtiyarova G.A., Yarmatova Sh.Z., Hafizova D.B., Bakhramova Sh. U. Adverse outcomes of assisted reproductive technologies in women with miscarriage in the presence of antiphospholipid antibodies (overview) “Frontiers in Bioscience-Landmark” Vol. 27. Issue 1. 2022. P. 129-134. |
| [14] | Yarmatova Sh.Z., Ikhtiyarova G.A. Early pregnancy loss after treatment with assisted Reproductive technologies. “Asian journal of Pharmaceutical and biological research” Vol. 11. Issue 1 2022. |



 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML








