-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(9): 2841-2847
doi:10.5923/j.ajmms.20251509.01
Received: Jul. 31, 2025; Accepted: Aug. 23, 2025; Published: Sep. 2, 2025

Evaluating the Impact of Experimental Thymectomy on the Cytogenetic Status of Bone Marro
Nuraliev Nekkadam Abdullaevich1, Sharipova Riboba G'ulomalievna2
1Vice-Rector at Urgench RANCH Technology University, Khorezm, Uzbekistan
2Basic Doctoral Student, Department of Allergology and Immunology, Bukhara State Medical Institute, Bukhara, Uzbekistan
Correspondence to: Sharipova Riboba G'ulomalievna, Basic Doctoral Student, Department of Allergology and Immunology, Bukhara State Medical Institute, Bukhara, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The aim of the study was to comparatively analyze cytogenetic changes in the bone marrow of outbred white rats against the background of experimental thymectomy, and to adequately assess the obtained results. All laboratory animals were divided into 4 representative experimental groups: Group 1 (Main Group): Outbred white rats that underwent thymectomy and did not receive Lactopropolis-AWL. Group 2 (Main Group): Outbred white rats that underwent thymectomy and received Lactopropolis-AWL. Comparison Group: Outbred white rats that underwent thymectomy and received ASD-2. Control Group: Intact outbred white rats that did not undergo thymectomy and did not receive probiotics or biostimulators. Studying the impact of thymectomy on cytogenetic investigations in bone marrow showed that while the proliferation activity of bone marrow cells in intact animals was 36.67%, after thymectomy, this parameter averaged 28.61%. This represents a significant decrease in activity by a factor of 1.28.
Keywords: Thymectomy, Bone marrow, Experimental studies, Cytogenetic changes
Cite this paper: Nuraliev Nekkadam Abdullaevich, Sharipova Riboba G'ulomalievna, Evaluating the Impact of Experimental Thymectomy on the Cytogenetic Status of Bone Marro, American Journal of Medicine and Medical Sciences, Vol. 15 No. 9, 2025, pp. 2841-2847. doi: 10.5923/j.ajmms.20251509.01.
Article Outline
1. Introduction
- It's well-known that cytogenetic study involves the qualitative and quantitative analysis of chromosome sets in an organism's cells. It examines the extent to which various external and internal factors affecting the body impact the human karyotype, identifying different pathological conditions [2,5,11].Chromosomes are identified as densely packed DNA strands that store information about the organism's genome. In humans, the nucleus of somatic cells contains 46 chromosomes (23 pairs). Of these, 22 pairs are autosomes, and 1 pair consists of sex chromosomes, which in turn determine a person's sex [3,6].The thymus is known to be one of the central organs of the immune system, where T-lymphocyte proliferation and differentiation occur, ensuring cellular immunity. Furthermore, the hormones produced by this organ are crucial for the body's overall function and its immune system. Its removal leads to deficiencies in cellular and humoral immunity parameters, resulting in secondary immunodeficiency [8,9].Experimental thymectomy was used to identify the connections between central immune system organs and the changes within them. Against this background, molecular changes in the bone marrow of laboratory animals were detected using cytogenetic analysis [10].White outbred rats were chosen for the experiments because, as mammals, their chromosome set is similar to that of humans (46 chromosomes – 23 pairs). By observing cytogenetic changes in them, one can assess the corresponding condition in humans.
2. Purpose of the Research
- The aim of the study was to comparatively analyze cytogenetic changes in the bone marrow of outbred white rats against the background of experimental thymectomy, and to adequately assess the obtained results.
3. Materials and Methods
- For the studies, a total of 80 three-month-old outbred white rats, weighing 160-180g, were randomly selected.In formulating the standard vivarium diet, we followed the feeding norms for experimental animals recommended by Nuraliev N.A. et al. [7]. Rules of biological safety and ethical principles for working with laboratory animals were observed during animal housing, euthanasia, and surgery [1].Thymectomy in outbred white rats was performed according to Victoria R. Rendell et al. [12]. This method reliably led to complete thymectomy, minimizing the time required for the operation and peri-procedural mortality rates.All laboratory animals were divided into 4 representative experimental groups:* Group 1 (Main Group): Outbred white rats that underwent thymectomy and did not receive "Lactopropolis-AWL," n=20.* Group 2 (Main Group): Outbred white rats that underwent thymectomy and received "Lactopropolis-AWL," n=20.* Comparison Group: Outbred white rats that underwent thymectomy and received ASD-2, n=20.* Control Group: Intact outbred white rats that did not undergo thymectomy and did not receive probiotics or biostimulators, n=20."Lactopropolis-AWL" is a probiotic consisting of an antagonistically active, live microbial mass of Lactobacillus casei 925 and Propionibacterium avidum, and a 1% dry extract of propolis. It is registered as a biologically active supplement (BAS) by the Ministry of Health of the Republic of Uzbekistan. This probiotic is a product of the Institute of Microbiology of the Academy of Sciences of the Republic of Uzbekistan and "AllWellLab" LLC. This probiotic positively affects the normal microflora of the large intestine and possesses antimicrobial, immunostimulating, and anti-inflammatory properties. The dosage of "Lactopropolis-AWL" administered to rats (0.3 ml/day per rat, for 14 days) was developed by Nuraliev N.A. et al. (2019), given in 2 doses per day (containing 2x10^7 CFU/ml strains).ASD-2 (Dorogov's Antiseptic-Stimulant) is a biologically active substance developed in the 1940s. ASD-2 is obtained from animal proteins through special thermal distillation. The appearance of ASD-2 is a liquid, black-yellow or reddish in color, with a characteristic pungent odor, easily soluble in water and other liquids. This substance has the following effects: it stimulates immune system activity, accelerates regenerative processes in the body, exhibits anti-inflammatory and antiseptic effects, detoxifies, and helps to balance hormonal levels. It is produced by Armavir Biofactory (Russian Federation). ASD-2 is registered as a veterinary product.For cytogenetic analysis, bone marrow from tubular bones was isolated during the euthanasia of outbred white rats, and cytogenetic changes were determined using a direct method. Metaphases in the preparation were observed under 200x magnification, metaphase plates were studied under 1000x magnification, and 15-25 cells containing metaphase plates were counted under the microscope for each sample, and the obtained numbers were analyzed. The number of dividing cells in the preparations was counted under the microscope and calculated using the mitotic index (MI) formula: MI = (Number of dividing cells / 1000) x 100. The following indicators were determined in the cytogenetic study: proliferative activity of bone marrow cells, number of metaphases and prophases, status of metaphase plates, identification of signs of pathological mitosis (spiralization, C-mitosis, aneuploidy, polyploidy), and cell death by necrosis and apoptosis types.The research material was statistically processed using parametric and non-parametric analysis methods. Data systematization and visualization of results were performed in Microsoft Office Excel 2016 spreadsheets, and statistical processing was carried out using IBM SPSS Statistics V. 26 software. The principles of randomization, representativeness, and evidence-based medicine were followed during the research.
4. Results and Discussion
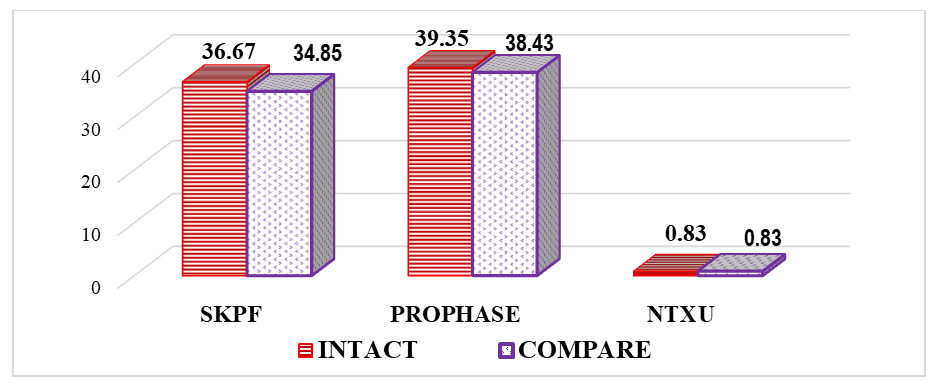
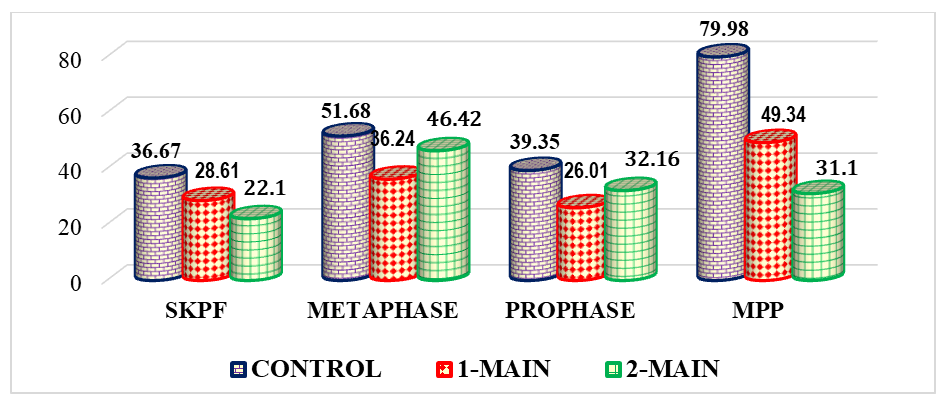
- To gain a clear understanding of all indicators obtained during the study, we analyzed the results of cytogenetic investigations performed on intact (control group) and thymectomized animals (main group 1).Our study on the impact of thymectomy on cytogenetic findings in bone marrow revealed that the proliferative activity of bone marrow cells in intact animals was 36.67%. After thymectomy, this parameter averaged 28.61%, representing a significant decrease in activity by a factor of 1.28.It is known that an increase in cell proliferative activity indicates the rate and extent of cell division. This occurs at a constant level during the physiological regeneration process of all tissues. Its reduction is observed during increased functional stress and tumor processes, while its decrease is seen in atrophic processes and various pathological exposures [4]. If we consider surgical removal of the thymus as a pathological exposure, then the decrease in bone marrow proliferation in thymectomized animals is assessed as a natural phenomenon. It is noteworthy that cell proliferation was inhibited by 8.06% in thymectomized animals compared to the control group.The study results showed that the number of metaphases in intact outbred white rats averaged 51.68%, while in thymectomized rats, it was 1.43 times lower, or a decrease of 15.54%.Considering that metaphase is the second phase of mitosis, during which chromosomes align at the cell's equator, their quantitative changes lead to mitotic disruption and chromosomal pathologies. Therefore, thymectomy causes a decrease in the number of metaphases, and a cytotoxic effect is also observed. A cytotoxic effect is the damaging effect of various external factors on the body's cells, leading to profound functional and structural changes within the cell and ultimately its death. This condition also applies to the cells of the bone marrow, another central organ of the immune system.Along with the number of metaphases, the number of prophases in bone marrow cells was also investigated. Prophase is the first phase of mitosis, during which DNA molecules in the cell nucleus condense and spiralize, forming compact chromosomes. Each chromosome consists of 2 DNA molecules linked by centromeres. At this stage, the nuclear envelope breaks down, and chromosomes lie dispersed in the cytoplasm. In animal cells, centrioles begin to move towards opposite poles of the cell.In the conducted study, the number of prophases in bone marrow cells varied. In intact laboratory animals, this parameter was 39.35%, whereas in thymectomized animals, this parameter was significantly lower by 1.51 times, or by 13.34% (Figure 1).
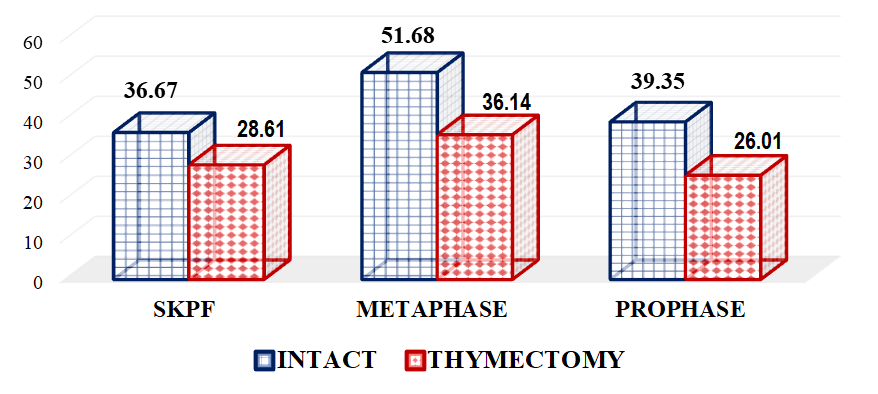 | Figure 1. Comparative indicators of cytogenetic studies in the bone marrow of thymectomized outbred white rats, % (BMPA – bone marrow cell proliferative activity) |
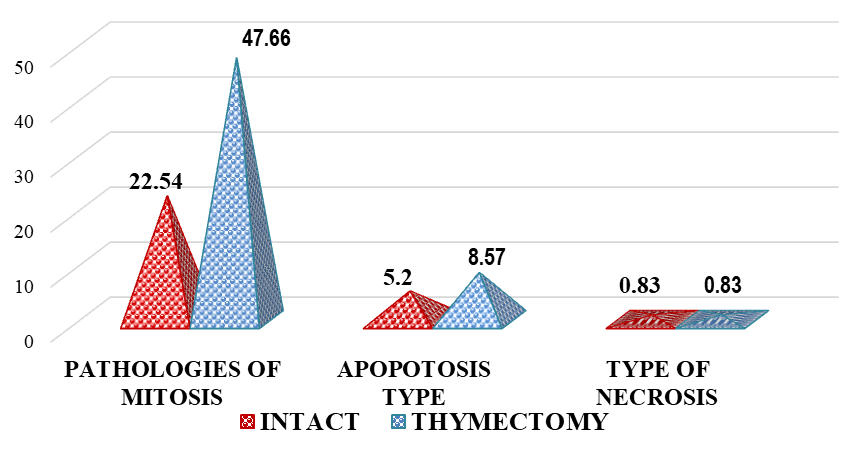 | Figure 2. Comparative indicators of pathological mitosis in the bone marrow of thymectomized outbred white rats, % |
 | Figure 3. Comparative indicators of cytogenetic changes in bone marrow cells after thymectomy, % (BMPA – bone marrow cell proliferative activity, MPNP – metaphase plate without pathology) |
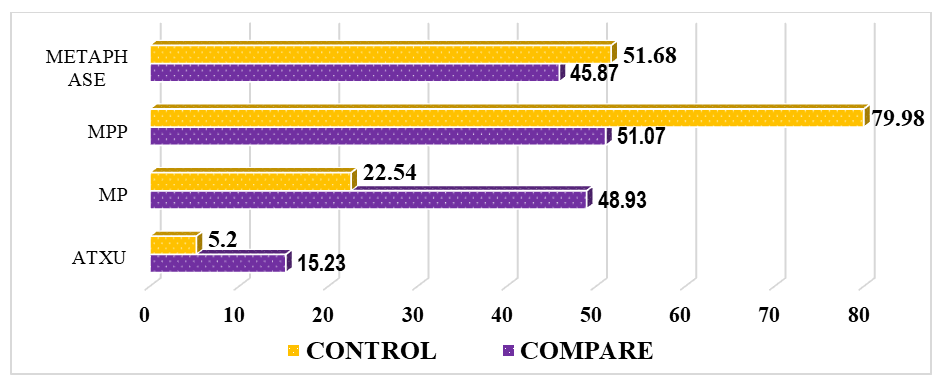
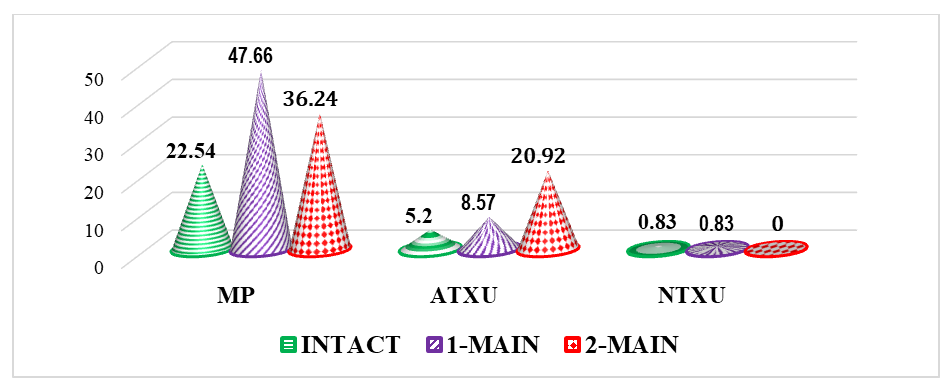 | Figure 4. Comparative parameters for detecting cell pathologies in bone marrow after thymectomy, % (MP – mitotic pathologies, ATCD – apoptotic type cell death, NTCD – necrotic type cell death) |
5. Conclusions
- It was found that the proliferative activity of bone marrow cells decreased by 1.28 times in thymectomized animals compared to intact ones, metaphases by 1.43 times, and prophases by 1.51 times. This indicates that thymectomy leads to a decrease in the proliferative activity of bone marrow cells and various cytogenetic changes, thus proving that thymectomy has a direct negative impact on bone marrow cells.In thymectomized outbred white rats, the instance of metaphase plates without pathology decreased by 1.62 times, which further proves the negative molecular-level impact of thymectomy on bone marrow cells. Mitotic pathologies increased by 2.11 times, and apoptosis increased by 1.65 times, while cell death of the necrotic type showed no significant difference between groups.In laboratory animals that underwent thymectomy and biocorrection, the proliferative activity of bone marrow cells decreased by 1.66 times. In contrast, the numbers of metaphases and prophases increased by 1.11 times and 1.20 times, respectively. While metaphase plates without pathology decreased by 2.57 times, mitotic pathologies decreased by 11.42%, and an increase in apoptosis (12.35%) was also noted. These results indicated that the "Lactopropolis-AWL" BAS did not demonstrate a clear significant effect.In the Comparison Group (thymectomy + ASD-2), the number of metaphases decreased by 1.13 times compared to intact animals, and the percentage of metaphase plates without pathology was 1.57 times lower. Mitotic pathologies remained high (a 2.17-fold difference). The increase in apoptosis also applied to the comparison group (a 2.93-fold difference). Thus, the cytogenetic changes in the bone marrow of laboratory animals in the comparison group were relatively weak, the cytotoxic effect was less developed, no structural changes in chromosomes were detected, and the high rate of cell death by apoptosis was acknowledged as potentially leading to DNA changes.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML