-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(8): 2660-2664
doi:10.5923/j.ajmms.20251508.52
Received: Jun. 24, 2025; Accepted: Jul. 19, 2025; Published: Aug. 15, 2025

Risk Assessment of Reproductive Losses in the First Trimester of Pregnancy: A Logistic Regression Model Based on IL-10
Muyassar Yaqubova1, Ziyoda Muminova2, Malika Yuldasheva1, Nargiza Gaipova1
1Tashkent State Medical University, Tashkent, Uzbekistan
2DSc, Associate Professor, Tashkent State Medical University, Tashkent, Uzbekistan
Correspondence to: Muyassar Yaqubova, Tashkent State Medical University, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Early identification of the risk of reproductive loss (spontaneous abortion) in the first trimester of pregnancy is crucial for preserving maternal health. This study is aimed to evaluate the predictive potential of interleukin-10 (IL-10), leukocyte, and monocyte levels in assessing the risk of spontaneous abortion. A total of 94 pregnant women participated in the study, of whom 28 experienced spontaneous abortion in the first trimester. IL-10 levels were significantly lower, while leukocyte and monocyte levels were significantly higher in women who experienced spontaneous abortion. A logistic regression model was constructed based on these parameters. The area under the ROC curve (AUC) was 0.88, indicating high diagnostic accuracy of the model. Calibration analysis confirmed a good agreement between the model’s predictions and actual clinical outcomes. The results suggest that IL-10, leukocyte, and monocyte levels may serve as effective prognostic markers for assessing the risk of spontaneous abortion in early pregnancy.
Keywords: Pregnancy, Spontaneous abortion, IL-10, Leukocytes, Monocytes, Logistic regression
Cite this paper: Muyassar Yaqubova, Ziyoda Muminova, Malika Yuldasheva, Nargiza Gaipova, Risk Assessment of Reproductive Losses in the First Trimester of Pregnancy: A Logistic Regression Model Based on IL-10, American Journal of Medicine and Medical Sciences, Vol. 15 No. 8, 2025, pp. 2660-2664. doi: 10.5923/j.ajmms.20251508.52.
Article Outline
1. Introduction
- Currently, reproductive losses (spontaneous termination of pregnancy) are a pressing issue for the healthcare system and society, particularly in the context of declining natural population growth rates [1]. It should be noted that spontaneous pregnancy termination (spontaneous abortion) is one of the most common complications during pregnancy. The first trimester of pregnancy (up to 12 weeks) is of particular importance, as this is when all the main physiological and immunological mechanisms for the normal progression of pregnancy are formed. Consequently, pathologies during this period can lead to significant negative consequences for the course of pregnancy and fetal development. Therefore, it is precisely these early stages of pregnancy that are the focus of current scientific research [2]. According to the literature, habitual miscarriages account for 5-20% of miscarriages. One of the most important aspects is that 75-80% of all fetal losses occur in the first trimester of pregnancy [3]. One of the important aspects is that an incorrectly developed pregnancy is considered a form of abortion. However, unlike spontaneous miscarriage, it is not accompanied by spontaneous cleansing of the uterine cavity [4]. As noted by a group of experts of the European Society for Human Reproduction and Embryology in 2020, the diagnosis of miscarriage is advisable only in cases where the embryo is located in the uterine cavity. Dynamic monitoring of the level of chorionic gonadotropin in the first trimester of pregnancy is of great diagnostic importance in assessing the course of pregnancy. With early reproductive losses, the concentration of this hormone in blood serum is usually below normal [8]. Also, in the early stages of pregnancy, one of the most informative methods that allows for diagnosis even before the appearance of clinical signs is the results of ultrasound examination [5]. The high frequency of miscarriage, the absence of a steady trend towards a decrease in this indicator, as well as the absence of reliable pathogenetic biomarkers for early diagnosis and prognosis, determined the topic and purpose of this scientific work. Therefore, the development of highly informative methods for determining the risk of reproductive loss in the first trimester is of great practical importance.These methods allow timely implementation of the following:• ultrasound examination of the pelvic organs;• Assessment of the hCG level;• and therapeutic measures as needed.As a result, this approach serves to preserve women's reproductive health, improve fertility, and increase the birth rate.
2. Purpose of the Study
- Purpose of the study is to develop of an assessment method for determining the risk of developing reproductive losses in the first trimester of pregnancy.
3. Materials and Methods
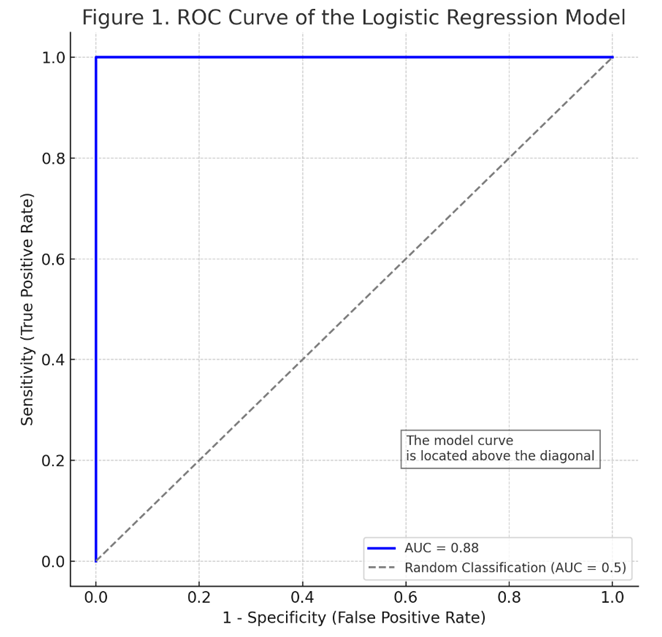
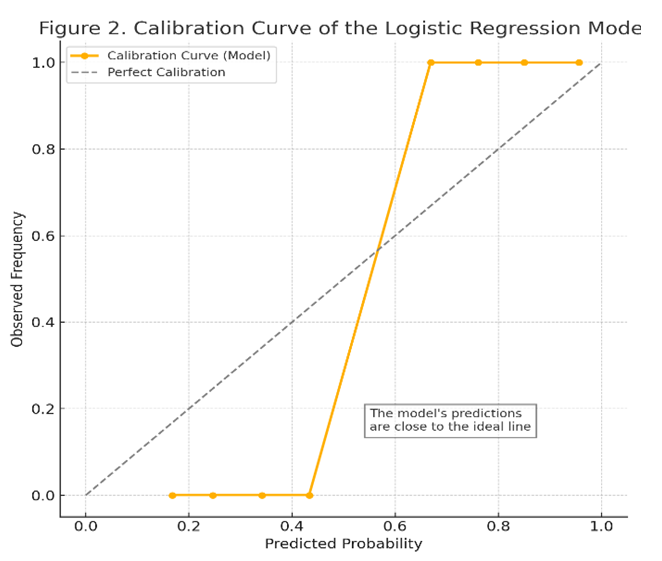
- This study was conducted in 2024-2025 at the Interdistrict Perinatal Center No. 9 of the city of Tashkent, where the following materials were analyzed from 94 pregnant women: blood serum, complete blood sample, and medical records of inpatient patients. Age of women: from 18 to 40 years. The study participants were divided into two groups: Main group (n=66): women admitted to the hospital at an early stage of pregnancy (5-12 weeks) with a risk of miscarriage. Subsequently, the following clinical diagnoses were confirmed: "Underdeveloped pregnancy" and chorionic pathologies; "Spontaneous abortion." Control group (n = 30): Women with normal, healthy pregnancy. During pregnancy, they were constantly monitored in the women's clinic.Inclusion criteria for the study:• Gestational age - from 6 weeks to 12 weeks;• Singleton pregnancy;• First episode of threatened miscarriage;• Absence of anatomical anomalies of the uterus;• Absence of severe somatic or serious gynecological diseases;• Written consent provided by the participant.The study involved 94 women, of whom 28 (29.8%) experienced spontaneous abortions in the first trimester during the observation period, while the remaining 66 had continued pregnancies and were deemed viable after 12 weeks. A single venous blood sample was taken from all participants between 5-12 weeks of gestation. Blood samples were collected in the morning, on an empty stomach, from the brachial vein of the pregnant women participating in the study. The concentration of IL-10 cytokine in the blood was measured using enzyme-linked immunosorbent assay (ELISA) method (in pg/ml). The total leukocyte count and monocyte count were determined through complete blood count and differential leukocyte formula, expressed in units of ×109/l, respectively. Subsequently, the progression of pregnancy during the first trimester was monitored in the participants, and the relationship between the obtained immune parameters and the occurrence of spontaneous miscarriage or continuation of pregnancy was analyzed.In the statistical analysis, univariate comparisons were first conducted, and the differences between groups with and without miscarriage were assessed using t-test and Mann-Whitney U-test. Subsequently, a multivariate logistic regression analysis was performed to examine whether IL-10, leukocytes, and monocytes independently had a significant influence as factors leading to spontaneous abortion. A simple explanation for the logistic regression model: the outcome variable is the termination of pregnancy with spontaneous miscarriage during the first trimester (binary: 1 - miscarriage occurred, 0 - pregnancy continued). The predictive equation of the model was expressed in the form logit (P). Coefficients β (beta) for model parameters, their standard errors (S.E.), Odds ratio (OR) and its 95% confidence interval (CI) and p-values for each indicator were calculated. To assess the discrimination capabilities of the model, a ROC curve was constructed, and the value of the area (AUC) under it was determined. In order to verify the calibration, the correspondence of the probabilities obtained by the model to the actual results was analyzed using the calibration curve (reliability diagram); the model's compatibility was also checked using the Hosmer-Lemeshow test, and the model was considered well calibrated at p>0.05. All calculations and graphical representations were performed using SPSS 26.0 and MedCalc.
4. Result
- During the study, the following statistical results were obtained:Age of women: The average age of women in the main group was 28 (25; 34) years old; In the control group, this indicator was 28 (25; No significant age difference was found between the groups (p = 0.44, U = 1055.5). 51.56% of women in the main group (95% confidence interval: 39.58-63.37%) and 53.33% of women in the control group (95% CI: 36.14-69.77%) had no birth history. No statistical differences were found between these indicators between the groups. Significant differences were observed in some biological indicators between pregnancies that ended with miscarriage and continued pregnancies.In women with spontaneous abortion, the level of IL-10 was significantly lower, with a median value of 30.5 pg/ml (Q25-Q75: 25.1-37.8), while in the group with prolonged pregnancy, the median was 49.0 pg/ml (Q25-Q75: 40.2-58.6) (p<0.001). On the contrary, the number of leukocytes was higher in cases ending in miscarriage, and their average concentration was 9.8±1.6 ×109/l, which is significantly higher (p=0.003) than in prolonged pregnancies (7.9±1.3 ×109/l). The absolute number of monocytes was also slightly higher in the descending group (0.53±0.15 ×109/l in the control group compared to an average of 0.72±0.20 ×109/l; p=0.021).The multifactorial logistic regression model confirmed the influence of these three indicators on the risk of independent decline. The logistic equation has the final form as follows:
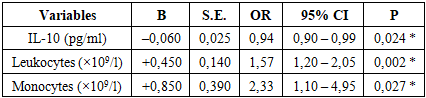
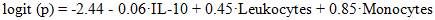 Here logit (p) is the logarithmic probability (logistic function) of the occurrence of a drop; IL-10 - the level of cytokine IL-10 in maternal blood (pg/ml); Leukocytes - the total number of leukocytes in the blood (×109/l); Monocytes - the number of monocytes in the blood (×109/l). When the β-coefficients in the equation are positive, an increase in the variable increases the risk of decline, and when negative, it reduces the risk. For example, an IL-10 level of β=-0.06 means that an increase in IL-10 by one unit reduces the likelihood of a drop. All parameters of the logistic regression model are presented in Table 1. The IL-10 indicator in the model was negative, its β = -0.06±0.025, OR = 0.94 (95% CI 0.90-0.99), p = 0.024. For the number of leukocytes, β = +0.45±0.14, which indicates an increase in the probability of decrease by approximately 1.57 times with each 1×109/L increase (OR = 1.57; 95% CI = 1.20-2.05; p = 0.002). The number of monocytes was also statistically significant (β = +0.85±0.39), and with an increase in the number of monocytes, the risk of miscarriage increased (OR = 2.33; 95% CI = 1.10-4.95; p = 0.027). For the intercept (β0) model, it is -2.44, which reflects the base probability of a decrease when other variables are averaged.
Here logit (p) is the logarithmic probability (logistic function) of the occurrence of a drop; IL-10 - the level of cytokine IL-10 in maternal blood (pg/ml); Leukocytes - the total number of leukocytes in the blood (×109/l); Monocytes - the number of monocytes in the blood (×109/l). When the β-coefficients in the equation are positive, an increase in the variable increases the risk of decline, and when negative, it reduces the risk. For example, an IL-10 level of β=-0.06 means that an increase in IL-10 by one unit reduces the likelihood of a drop. All parameters of the logistic regression model are presented in Table 1. The IL-10 indicator in the model was negative, its β = -0.06±0.025, OR = 0.94 (95% CI 0.90-0.99), p = 0.024. For the number of leukocytes, β = +0.45±0.14, which indicates an increase in the probability of decrease by approximately 1.57 times with each 1×109/L increase (OR = 1.57; 95% CI = 1.20-2.05; p = 0.002). The number of monocytes was also statistically significant (β = +0.85±0.39), and with an increase in the number of monocytes, the risk of miscarriage increased (OR = 2.33; 95% CI = 1.10-4.95; p = 0.027). For the intercept (β0) model, it is -2.44, which reflects the base probability of a decrease when other variables are averaged.
|
5. Discussion
- The results of this study showed that immunological indicators such as IL-10, leukocytes, and monocytes, collectively, can be useful in predicting the risk of reproductive loss in the early stages of pregnancy. In the identified model, a decrease in the level of IL-10 was confirmed as an independent factor increasing the risk of miscarriage. This finding corresponds to previous scientific data - for example, Kaislasuo et al. (2020) showed that the concentration of IL-10 in healthy pregnancies was consistently high during the first trimester, and significantly lower in pregnancies that ended in miscarriage [6]. IL-10 is a powerful anti-inflammatory cytokine, and it has been noted in various studies that its deficiency can be harmful to the fetus [7,9,10]. In our study, it was revealed that a low level of IL-10 is associated with miscarriage, which can be a consequence of a disruption of immune balance (Th2/Th1 balance) during pregnancy. In our study, it was confirmed that leukocytosis (increase in white blood cells) in peripheral blood can also be an indicator of an increased risk of miscarriage in the first trimester. A high leukocyte count usually indicates an inflammatory or stressful state in the body.Al-Husban and others came to the same conclusion: in women with a leukocyte count above 10×109/l in the first trimester of pregnancy, the risk of miscarriage and premature birth was significantly higher [1]. As we observed, the average leukocyte count in women with miscarriages was higher, and in the logistical model, an increase in the number of leukocytes was also associated with an OR of ~1.6 (a 60% higher risk). This result indicates that successful pregnancy requires normal and controlled immunity, and excessive inflammation in many cases can create a negative environment for the fetus.Another important factor in our study - the number of monocytes - was also associated with the risk of miscarriage, which confirms the participation of the innate link of the immune system in this process. Monocytes and macrophages perform regulatory and protective functions in the decidual tissue of the uterus during normal pregnancy. If the balance of monocytes and their subpopulations changes, this can interfere with fetal development [5]. For example, although alternatively activated (M2 phenotype) monocytes usually provide protection and tolerance for the fetus, Farzalieva et al. (2022) found a sharp increase in the number of this type of monocytes in high-risk pregnancies (threatened miscarriage) and proposed it as a negative prognostic sign. In our study, the increase in the total number of monocytes showed an independent correlation with miscarriage. In this case, an increase in the number of monocytes may possibly reflect an increase in inflammatory mediators (such as TNF-α, IL-6) and an imbalance of the immune system.At the same time, since the number of monocytes depends on the total number of leukocytes, their independent influence can be relatively small. In broader studies, not only the number of monocytes, but also the active phenotype (for example, the proportion of CD163-expressive monocytes) can be taken into account, and the model can be further improved [4]. It should be noted that the developed logistics model has high discrimination capability (AUC ~0.88) and good calibration within the framework of internal validation. However, before introducing the model into clinical practice, it is necessary to confirm it in independent external data.According to our existing results, the combination of immunological biomarkers (IL-10) and simple laboratory parameters (leukocytes and monocytes) can serve to predict the risk of miscarriage with high accuracy. For comparison, in other studies, nomogram-models containing many factors were proposed, for example, Ding et al. (2025) created a model predicting miscarriage based on 10 factors in women with a history, achieving AUC ~0.72 [3]. Since our model includes indicators directly related to immune mechanisms, it is possible that it provided high sensitivity in the detection of prolapse. Of course, for the clinical applicability of the model, it will be necessary to test it on a wider population, as well as to clarify the cross-section points. Laboratory equipment for measuring IL-10 levels is currently available, and it is a relatively inexpensive test; leukocytes and monocytes are detected in a simple blood test. Therefore, this model can be convenient for practical application and economically effective. For example, if, according to model calculations, the probability of miscarriage exceeds 50% in a woman who donated blood at the first trimester of pregnancy, this can be a reason for more careful observation of the patient, inpatient treatment, or other preventive measures. In addition, if the probability is very high, it will be possible to provide timely advice and psychological support to the patient regarding the preservation of pregnancy.
6. Conclusions
- The logistic regression model, based on the level of cytokine IL-10, the number of leukocytes, and the number of monocytes in the mother's blood in the first trimester of pregnancy, allows for a reliable assessment of the risk of spontaneous abortion. The results of the model show that a low level of IL-10 and an increase in the number of leukocytes and monocytes significantly increase the probability of miscarriage (p<0.05). The combined assessment of all three indicators gives the model high discriminatory ability (AUC ~88%). The probabilistic predictions derived by the model well matched the real clinical results, which confirms its calibration.This approach can be useful in clinical practice for identifying high-risk groups among pregnant women in the first trimester and providing them with early assistance measures. In future research, it is advisable to validate the model in larger and more diverse populations, as well as to improve the accuracy of prognosis by adding additional biomarkers to the list of immunological factors.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML