-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(8): 2586-2589
doi:10.5923/j.ajmms.20251508.35
Received: Jun. 21, 2025; Accepted: Jul. 16, 2025; Published: Aug. 6, 2025

The Role of Thrombodynamics in the Diagnosis of Ulcerative Gastro-Duodenal Bleeding
Bobokulov A. U., Daminov F. A.
Samarkand State Medical University, Samarkand, Uzbekistan
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Despite the long history, the problem of treating gastroduodenal bleeding of various origins in emergency surgery does not lose its relevance. The most common cause of them is gastric ulcer and 12-denum ulcer, less often bleeding gastric cancer, Mallory-Weiss syndrome. Mortality in gastroduodenal bleeding of ulcerative genesis has not yet had a noticeable tendency to decrease and, according to various authors, ranges from 8 to 30%, reaching maximum values in elderly and senile patients. This is due to the high level of forced operations performed on an emergency basis at the height of profuse bleeding - from 20 to 53%.
Keywords: Gastroduodenal bleeding, Thrombodynamics, Gastric ulcer, Surgical interventions, Coagulogram
Cite this paper: Bobokulov A. U., Daminov F. A., The Role of Thrombodynamics in the Diagnosis of Ulcerative Gastro-Duodenal Bleeding, American Journal of Medicine and Medical Sciences, Vol. 15 No. 8, 2025, pp. 2586-2589. doi: 10.5923/j.ajmms.20251508.35.
1. Topicality
- Taking into account the fact that gastric bleeding can be the first manifestation of gastric cancer (especially its ulcerative forms), the treatment of this category of patients is carried out by emergency surgeons [1]. The complexity of this problem also lies in the fact that the clinical picture and endoscopy data of bleeding gastric cancer can be disguised as a chronic ulcer, directing surgeons in the wrong direction. In addition to peptic ulcer disease, gastroduodenal bleeding can be a consequence of erosive-ulcerative lesions of the upper gastrointestinal tract, complicating the course of emergency conditions of various profiles, among which severe burn injury (Kurling's ulcers), polytrauma, severe neurosurgical pathology (Cushing's ulcers) should be noted. Mortality among patients with emergency conditions complicated by erosive-ulcerative gastroduodenal bleeding remains at a high level and ranges from 40 to 90% [2]. As studies have shown, the pathogenesis of acute erosive-ulcerative lesions of the upper gastrointestinal tract in emergency conditions is multifactorial, it is based on deep disturbances of homeostasis due to traumatic shock, surgical aggression, severe intoxication and multiple organ failure syndrome with impaired liver and kidney function [3]. The problem of treating gastroduodenal bleeding of various origins in a multidisciplinary emergency hospital, where patients with the listed profile of diseases and injuries are concentrated, requires the creation of a unified program aimed at both reducing mortality in this pathology and preventing cases of gastroduodenal bleeding in the hospital in patients with emergency conditions [4].Purpose. To study the characteristics of the thrombogenesis process in patients with ulcerative gastroduodenal bleeding taking various direct oral anticoagulants (POAC) and ulcer irrigation with captofer using the Thrombodynamics test.
2. Materials and Methods
- A total of 48 patients aged 41–60 years were followed up with a diagnosis of ulcerative gastroduodenal bleeding. 36 (73.9%) were men and 12 (26.1%) were women. 18.2% were admitted with bleeding in the first 6 hours, 17.8% in the period from 6 to 12 hours, 29.1% in 12–24 hours, and 34.9% after 24 hours, i.e. most patients were admitted to the clinic later than 24 hours due to developed hemodynamic disorders [5]. Chronic gastric ulcer was a source of bleeding in 36.9% of cases. duodenal ulcer – in 59.4%. Bleeding from concomitant chronic gastric and duodenal ulcers was observed in 3.7%. All patients were divided into 2 groups:Group I (n=22) consisted of patients with gastrointestinal bleeding who took oral anticoagulant Plavix.Group II (n=36) consisted of patients who took anticoagulants and additionally irrigated the ulcer with a capofer solution and applied electrocoagulation.Blood sampling and plasma preparation. Blood sampling from patients was carried out immediately before taking the next dose of the anticoagulant (after at least 5 half-lives, when the concentration of the drug in the blood plasma took a "steady-state" state and a stable pharmacodynamic effect developed). Minimal impact on test results in healthy donors: Vacuette plastic 4.5 ml 3.8% citrate [6]. The ratio of blood volume to sodium citrate in a test tube is 9:1. Blood samples were centrifuged at 1600 g for 15 min, the upper part of the obtained plasma was taken, and then platelet-free plasma used for analysis was obtained by additional centrifugation for 5 min at 10000 g. The procedure was performed at room temperature.Research method. A prepared sample of platelet-free plasma in the volume of 120 ml is placed in a microtube with a control solution I for subsequent incubation at a temperature of 37°C for 3 minutes. The growth of a fibrin clot from the end of the activator insert in the cuvette channel is recorded by the device in the mode of sequential photography using the darkfield method for 30 minutes [7].To assess the hypo- and hypercoagulable state of the hemostasis system, the efficacy of the dose of anticoagulants, the "Thrombodynamics Recorder T-2" was used - an early test for the control of plasma hemostasis. A fibrin clot formed from activation by a tissue factor in an unstirred thin layer of the plasma under study is recorded by red LEDs in the temperature-controlled chamber by the optical method. Next, computer processing of data is performed. The following indicators are evaluated: – growth retardation of the Tlag clot;– the time interval from the moment of contact with the thromboplastin to the moment of the beginning of the formation of a fibrin clot (0.7–1.4 minutes); – initial growth rate of the clot VI – the initial segment of the graph of the dependence of the clot size on time (from 2 to 6 minutes from the beginning of the clot growth) (36–54 μm/min); – the stationary growth rate of the clot Vst is calculated from the slope of the approximating stationary region of the graph of the clot size versus time (in the range from 15 to 25 minutes from the beginning of the clot growth) (19–30 μm/min); – clot size (MS);– integral indicator characterizing the growth process of the clot as a whole (800–1200 μm); – clot density (CP) characterizes the density of the formed clot (15000–32000 conventional units); – the appearance of spontaneous clots (normally absent);– the time of the formation of fibrin clots away from the end of the plastic insert (activator).And also studied the parameters of the blood coagulation system. The parameters of the blood coagulation system were assessed using standard (routine) coagulological tests: activated partial thromboplastin time (APTT), thrombin time (PTT), antithrombin III (AT III) activity, international normalized ratio (INR) value, fibrinogen content on an automatic coagulometer CA-500 (Sysmex, Japan) using Siemens (Germany) reagents were determined [8]. The level of D-dimer was measured on an immunochemiluminescence analyzer Immulite 2000 (Siemens Healthcare Diagnostics Inc., USA), reagents were obtained from Siemens. The amount of hemoglobin, hematocrit and platelets in the blood was calculated on the BC-5300 hematology analyzer (Mindray, China) [9].
3. Results of the Study
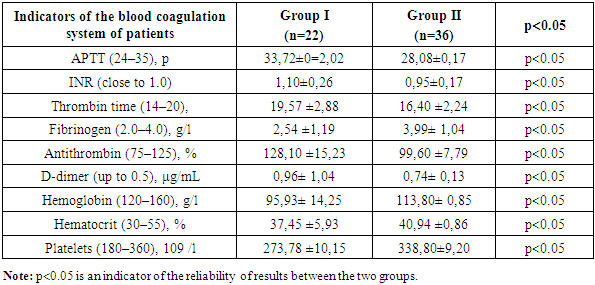
- The results of the study showed that in patients of group 1 who took only Plavix and in the second group who took oral anticoagulants and ulcer irrigation with Caprofer solution was used, the picture of the coagulogram was as follows: APTT was 33.72±0=2.02, in the second group this indicator was 28.08±0.17, in the first group the INR was 1.10±0.26, in the second group it was significantly 0.95±0.17. Thrombin time in group 1 was 19.57 ±2.88, in the second group, it was in the range of 16.40 ±2.24. The amount of fibrinogen in the first group was 2.54 ±1.19, in the second group 3.99±1.04. Antithrombin in the first group was determined in the amount of 128.10±15.23, and in the second group it significantly amounted to 99.60 ±7.79, which indicates the effectiveness of the method used together with an anticoagulant. The amount of D-dimer in group 1 was 0.96±1.04, in group 2 0.74±0.13. This result indicates that patients in group 1 have a high risk of rebleeding. The level of hemoglobin in the first group was 95.93±14.25, in the second group 113.80± 0.85. Based on the level of hemoglobin, it can be said that repeated bleeding was not observed in the second group. The hematocrit level was 37.45 ±5.93 in the 1st group and 40.94 ±0.86 in the second group within the normal range. Platelet counts in the first group and in the second group were within normal limits (273.78 ±10.15 and 338.80±9.20, respectively).
|
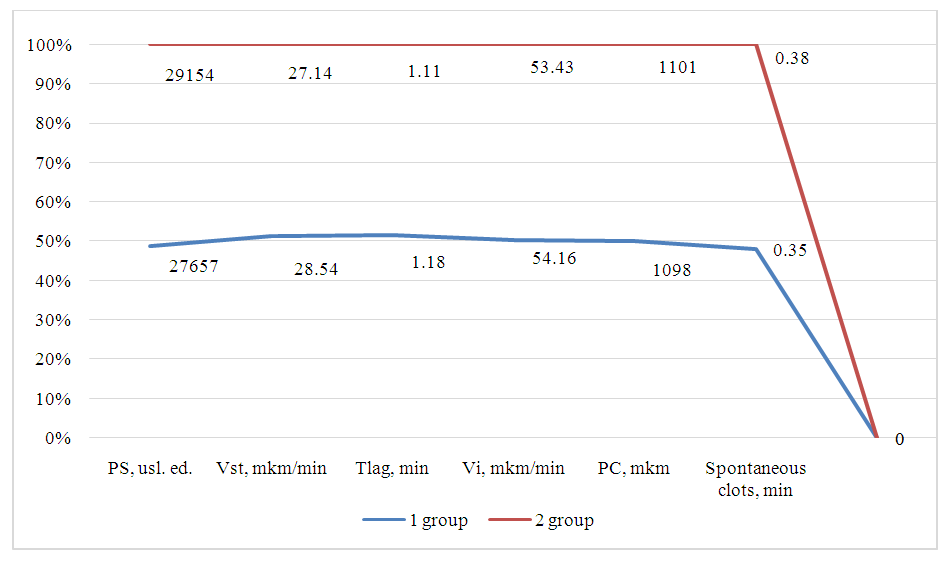 | Figure 1. Thrombodynamic parameters in the study groups |
4. Inference
- Thus, according to the results of the coagulogram, it can be analyzed that patients of group 1 have a significant risk of recurrent bleeding. For whom the use of ulcer irrigation with capofer or electrocoagulation is recommended. In group 1, this indicator was 0.35 min, while in group 2 there was no spontaneous thrombosis (0 min). This may indicate a more pronounced tendency to hypercoagulability in patients in group 1. Despite the general similarity of most parameters, the revealed differences, in particular, the presence of spontaneous thrombogenesis in group 1, may be of clinical significance and require further analysis.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML