-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(8): 2577-2581
doi:10.5923/j.ajmms.20251508.33
Received: Jun. 7, 2025; Accepted: Jul. 28, 2025; Published: Aug. 6, 2025

Clinical and Laboratory Features of Drug-Sensitive and Drug-Resistant Forms of Tuberculosis in Children and Adolescents
Abdusalomova Makhliyo Ismailovna
Assistant, Department of Phthisiology, Andijan State Medical Institute, Andijan, Uzbekistan
Correspondence to: Abdusalomova Makhliyo Ismailovna, Assistant, Department of Phthisiology, Andijan State Medical Institute, Andijan, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This study presents a comparative analysis of clinical and laboratory characteristics in 182 children and adolescents diagnosed with either drug-sensitive or drug-resistant forms of tuberculosis (TB). Drug-resistant tuberculosis (DR-TB) was more frequently observed in pulmonary forms and was associated with more severe clinical courses, higher levels of bacterial excretion, and increased rates of complications (including pleurisy and cardiopulmonary failure). Hyperergic reactions to Diaskintest and low rates of positive Mantoux test reactions in DR-TB patients indicate distinct immune responses. Among resistant forms, multidrug-resistant TB (MDR-TB) and extensively drug-resistant TB (XDR-TB) predominated, especially among adolescents. Children with DR-TB more frequently exhibited symptoms of intoxication, anemia, and delays in physical and mental development. The findings emphasize the need for early detection and individualized diagnostic and therapeutic approaches, particularly in high-risk groups.
Keywords: Tuberculosis, Children, Adolescents, Drug resistance, MDR-TB, Diaskintest, Mantoux test, Clinical forms
Cite this paper: Abdusalomova Makhliyo Ismailovna, Clinical and Laboratory Features of Drug-Sensitive and Drug-Resistant Forms of Tuberculosis in Children and Adolescents, American Journal of Medicine and Medical Sciences, Vol. 15 No. 8, 2025, pp. 2577-2581. doi: 10.5923/j.ajmms.20251508.33.
1. Introduction
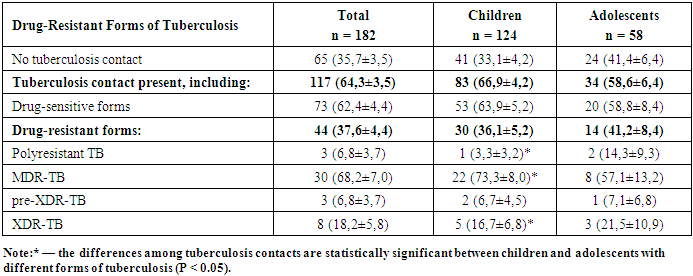
- Tuberculosis (TB) remains a significant health threat, especially among children. In 2023, 10.8 million TB cases were registered worldwide, with 12% occurring in children (WHO, 2024). Pediatric TB diagnosis remains challenging, with only 55% of cases being detected. In Russia, child health is protected at the state level, and mass BCG vaccination has been in place since 1949. Advances in molecular biology have improved diagnostic capabilities; however, prevention remains a key focus. Enhancing early detection and treatment methods continues to be a top priority in both medical practice and public health policy.According to Aksenova V.A. (2019), clinical characteristics of children and adolescents with drug-sensitive and drug-resistant TB show that TB remains a serious concern for the pediatric and adolescent population. In 2023, 10.8 million cases were registered globally, of which 12% were in children. TB symptoms in children are often nonspecific, which complicates diagnosis. Drug-resistant forms require more complex treatment regimens. Modern diagnostic methods, including PCR and interferon-based tests, facilitate early disease detection. Active case finding helps reduce severe TB forms, bacterial excretion, and destructive lung changes. Early diagnosis and effective treatment are key to reducing TB incidence and preventing complications [1].Drug-resistant TB in children is a serious issue linked to improper treatment and social factors. Symptoms are often nonspecific, which makes timely diagnosis difficult. In Saratov Oblast and Kazakhstan, TB incidence has declined due to early detection and preventive measures, but latent TB infection (LTBI) remains a reservoir for future disease. Modern diagnostic tools, including molecular-genetic tests and CT scans, enable timely detection. In Kazakhstan, adolescents are considered a high-risk group and undergo annual fluorography. Studies confirm the importance of skin tests for identifying LTBI and optimizing prevention strategies, which calls for updates to existing regulations [4,7].In Saint Petersburg (2015–2017), among children who developed TB in infectious foci, the majority had contact with patients with drug-sensitive (41.2%) and drug-resistant (13.7%) TB. Children with multidrug-resistant tuberculosis (MDR-TB) more often experienced severe disease, lacked BCG vaccination, and had identifiable social risk factors. Drug-resistant cases required individualized therapy that considered Mycobacterium tuberculosis (MBT) resistance. Laboratory studies showed high bacterial load and altered immune status in patients with MDR-TB, emphasizing the importance of early diagnosis and timely treatment adjustments [10].
2. Materials and Methods
- The aim of this study was to examine the clinical and laboratory characteristics of children and adolescents with different forms of tuberculosis, including both drug-sensitive and drug-resistant types. A retrospective analysis of 182 confirmed TB cases was conducted in patients aged from 1 month to 18 years undergoing inpatient treatment in a specialized TB hospital.Patients were categorized into four age groups:Infants (under 3 years),Preschool-aged (3–6 years),School-aged (7–14 years),Adolescents (15–18 years).Both drug-sensitive and drug-resistant TB cases were included. Drug resistance included mono-, poly-, multidrug (MDR), and extensively drug-resistant (XDR) types.Diagnosis was based on a combination of clinical, radiological, laboratory, and microbiological criteria, including:Tuberculin skin testing (Mantoux, Diaskintest),Imaging (chest X-ray, CT),Sputum and bronchial lavage smear and culture,Molecular genetic methods (PCR, GeneXpert, HAIN tests) for detection of Mycobacterium tuberculosis (MTB) and drug resistance.Clinical and laboratory data were analyzed with consideration of TB form and resistance pattern. Statistical analysis involved calculation of absolute and relative frequencies (%), means, and standard errors. Differences between groups were evaluated using Student’s t-test, with significance set at p<0.05.
3. Results
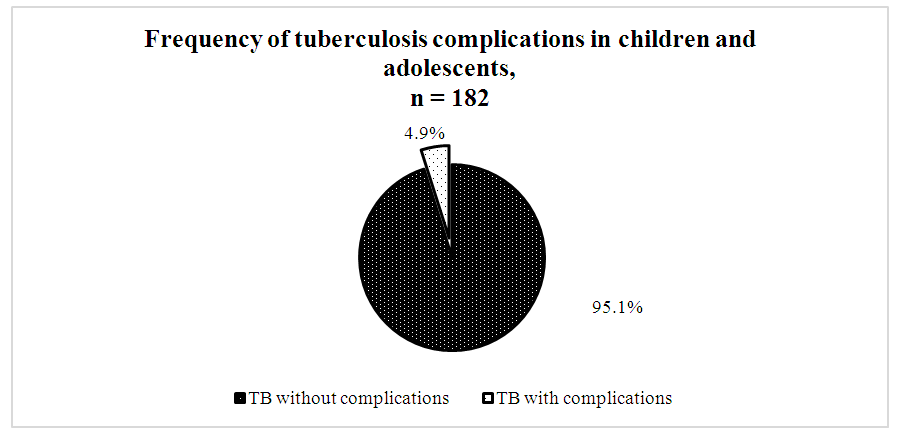
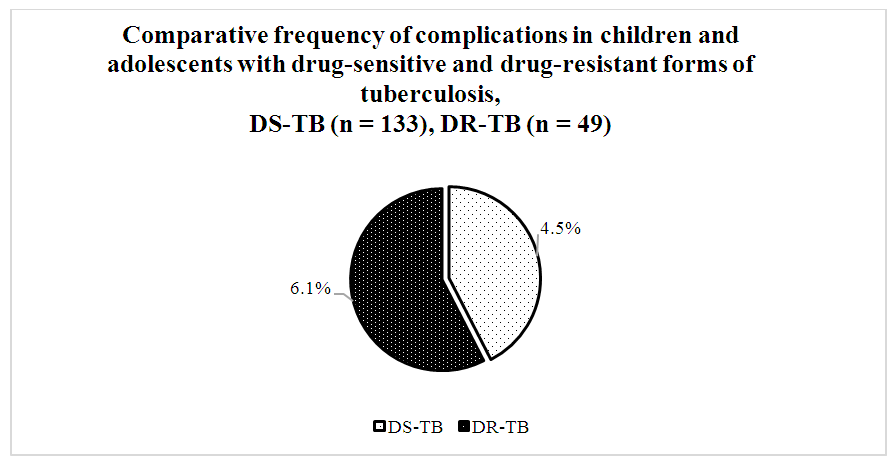
- A comparative analysis of 182 patients revealed significant differences between drug-sensitive and drug-resistant TB forms. Among contacts with TB patients, drug-resistant forms were 1.7 times less frequent (37.6±4.4%) than sensitive ones (62.4±4.4%, p<0.05). Hyperergic Diaskintest reactions were observed 2.6 times less often in drug-resistant TB (28.1±4.5%) compared to severe drug-sensitive TB (71.9±4.5%, p<0.05).Positive Mantoux reactions were 4.7 times less frequent in drug-resistant TB (17.5±4.7%) than in sensitive TB (82.5±4.7%, p<0.05).According to bacteriological studies, TB was diagnosed twice as often in patients with resistant forms (66.7±13.6%) than with sensitive forms (33.3±13.6%, p<0.05).TB FormsPulmonary drug-resistant TB was twice as common (38.8±6.9%) as pulmonary drug-sensitive TB (19.5±3.4%, p<0.05).Conversely, intrathoracic lymph node TB was more frequent in drug-sensitive forms (80.5±3.4%) than resistant ones (61.2±6.9%, p<0.05).Infiltrative TB was more prevalent in resistant cases (31.5±10.6%) than sensitive ones (3.8±3.7%, p<0.05). Generalized, disseminated, and focal forms were only observed in drug-sensitive TB.Disease Course and ComplicationsThe phases of dissemination and detection were significantly more frequent in drug-resistant forms (12.2±4.6%) compared to drug-sensitive forms (3.7±1.6%, p<0.05).As shown in Diagram 1, among the 182 patients studied, complications were recorded in 9 individuals (4.9%).
 | Diagram 1 |
 | Diagram 2 |
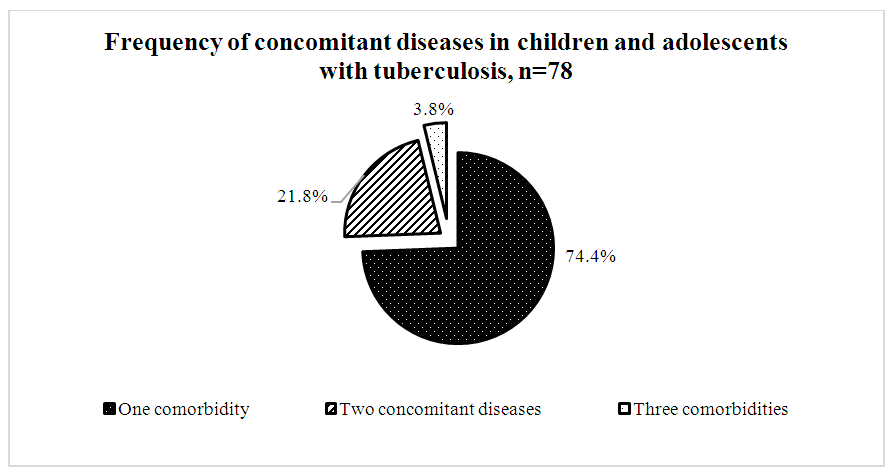 | Diagram 3 |
|
4. Discussion
- The results show that DR-TB in children and adolescents has a more severe course, fewer pronounced skin reactions, and more frequent complications and comorbidities. The higher frequency of drug-sensitive TB among contacts (62.4% vs. 37.6%) supports earlier findings [13] emphasizing early chemoprophylaxis to prevent resistance. The higher rate of hyperergic Diaskintest reactions in sensitive TB corresponds with observations by Khomenko (2019), who noted stronger tuberculin responses in active, drug-sensitive TB. Reduced Mantoux positivity in DR-TB may indicate immune hypo-responsiveness, consistent with Chesnov et al. (2020), who described immunological tolerance in MDR-TB. Infiltrative TB predominated in DR-TB, supporting Mirzaeva’s findings (2022) that link this form with high bacterial excretion.Dissemination phases were more common in resistant forms, aligning with Vasilieva et al. (2018), who reported delayed medical consultation and high bacillary load in DR-TB. Cardiopulmonary failure occurred only in DR-TB, confirming Egorova and Saveleva’s (2021) conclusions about the severity of resistant TB complications. MDR-TB (68.2%) and XDR-TB (18.2%) were most prevalent. WHO (2022) notes MDR-TB is more frequent in primary infection among children, while XDR-TB is typical in adolescents with prior treatment history.Developmental delays in DR-TB were more frequent, consistent with Kolesnikova et al. (2020), who highlighted metabolic disruption in pediatric drug-resistant TB.Intoxication symptoms were predominant in DR-TB, as also observed by Polyakova et al. (2020), who noted a polymorphic intoxication syndrome in MDR-TB.The findings indicate that drug-resistant tuberculosis in children and adolescents is marked by a more severe clinical course, higher rates of complications, immune dysregulation, and developmental impairments, emphasizing the need for early detection, differential diagnosis, and tailored treatment strategies.
5. Conclusions
- 1. TB was more commonly detected among schoolchildren and adolescents. Among preschoolers, girls were 2.2 times more likely to be affected; among schoolchildren, boys predominated.2. DR-TB more frequently presented in pulmonary forms, while sensitive TB was more often localized to intrathoracic lymph nodes.3. DR-TB was associated with more severe disease, more complications (e.g., cardiopulmonary failure), and more pronounced intoxication symptoms.4. Hyperergic Diaskintest reactions were significantly more frequent in DR-TB, underscoring its value in early diagnosis of drug-resistant TB in children.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML