-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(8): 2562-2569
doi:10.5923/j.ajmms.20251508.30
Received: Jun. 20, 2025; Accepted: Jul. 22, 2025; Published: Aug. 6, 2025

Association of Lys197Asn Polymorphism in the EDN1 Gene as a Risk Factor for Endothelial Dysfunction and Cerebrovascular Diseases
Inoyatova S. O.1, Adambaev Z. I.2, Madjidova Y. N.1, Boboev K. T.3, Abdukodirov E. I.1
1Tashkent State Medical University, Uzbekistan
2Urganch State Medical Institute, Uzbekistan
3Republican Specialized Scientific and Practical Medical Center of Hematology, Ministry of Health of the Republic of Uzbekistan
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Cerebrovascular diseases (CVDs) represent a significant medical and social problem. Therefore, studying genetic risk factors, such as the Lys197Asn polymorphism in the endothelin-1 (EDN1) gene, is crucial for understanding the pathogenesis and developing preventive strategies. Objective: To assess the association of the Lys197Asn polymorphism in the EDN1 gene with the development of endothelial dysfunction and cerebrovascular diseases. Materials and Methods: The study included 176 patients with CVDs (37 with confirmed stroke and 139 with pre-stroke cerebrovascular conditions) aged between 30 and 75 years. The control group consisted of 101 healthy residents of Uzbekistan with no risk factors or clinical manifestations of CVD. All participants underwent clinical examination and lipid profile analysis. Genotyping of the Lys197Asn polymorphism (rs5370) was performed by RT-PCR after DNA extraction from peripheral blood. The study protocol was approved by the local ethics committee. Results: A possible association was found between the Lys197Asn polymorphism in the EDN1 gene and the risk of developing CVD. The presence of the Asn allele and the Asn/Asn genotype was observed to be associated with an increased risk. However, the analysis of diagnostic indicators revealed high sensitivity (0.71–0.81) but low specificity (0.23–0.29), indicating limited prognostic value of this polymorphism for individual risk assessment. Conclusion: The Lys197Asn polymorphism in the EDN1 gene may be a potential risk factor for the development of cerebrovascular diseases, particularly in combination with the Asn allele. Further research is needed to clarify its role, considering gene–environment interactions and the ethnic background of patients.
Keywords: Lys197Asn polymorphism of the EDN1 gene, Endothelial dysfunction, Cerebrovascular diseases, Stroke
Cite this paper: Inoyatova S. O., Adambaev Z. I., Madjidova Y. N., Boboev K. T., Abdukodirov E. I., Association of Lys197Asn Polymorphism in the EDN1 Gene as a Risk Factor for Endothelial Dysfunction and Cerebrovascular Diseases, American Journal of Medicine and Medical Sciences, Vol. 15 No. 8, 2025, pp. 2562-2569. doi: 10.5923/j.ajmms.20251508.30.
1. Introduction
- Structural changes in the cerebral vascular bed are not the only contributors to the development of cerebrovascular diseases (CVDs); functional impairments of the vascular wall also play a crucial role. Currently, the vascular endothelium is considered not only a target organ for arterial hypertension, atherosclerosis, and type 2 diabetes mellitus, but also an effector in the pathogenesis of complications such as cerebrovascular diseases [1].The vascular endothelium is a heterogeneous structure with diverse functions and acts as an active metabolic system. By producing various biologically active substances, the endothelium directly participates in maintaining vascular tone, the atherothrombogenic potential of the vascular wall, regulation of platelet adhesion and aggregation, and demonstrates pro- and anticoagulant, as well as fibrinolytic activity. It is also involved in inflammation and angiogenesis [2]. Constantly in direct contact with the blood, the endothelium receives signals through humoral pathways (via blood-borne substances that bind to receptors on the luminal surface) and through direct interactions with blood cells or changes in shear stress (e.g., variations in linear blood flow velocity).The term “endothelial function” refers to the ability of the endothelium to regulate blood flow in microvessels, ensuring adequate tissue oxygenation and nutrient delivery [3]. “Endothelial dysfunction” is defined as the impaired ability of vessels to respond appropriately to stimuli. It is a key factor in the development of diseases such as atherosclerosis, hypertension, and preeclampsia [3,4].Endothelial cells lining the inner surface of blood vessels play an essential role in tissue respiration and metabolism. Under normal conditions, they are relatively quiescent but become rapidly activated in response to injury or pathological conditions that require neovascularization or angiogenesis [5]. Endothelial dysfunction is recognized as a universal mechanism in thrombosis, neovascularization, vascular remodeling, and activation of platelets and leukocytes, all of which are integral to the initiation and progression of cerebrovascular disease [6].There are four main types of endothelial dysfunction: vasomotor, hemostatic, adhesive, and angiogenic [7], which can manifest as either hypo- or hyperfunction [8]. Isolated dysfunctions are rare and usually associated with congenital (hypofunction) or acquired (hyperfunction) disorders. Typically, a combination of dysfunctions is observed, with clinical manifestations depending on the underlying disease. Further, we examine the role of endothelial dysfunction in stroke development, especially in the context of hypertension, type 2 diabetes mellitus, and atherosclerosis.Numerous studies have investigated the role of the endothelium in the development of hypertension (HTN), with particular focus on impaired nitric oxide (NO) synthesis, which leads to vascular dysfunction and plays a central role in HTN pathogenesis [3,9,10]. As a mediator of endothelium-dependent vasodilation, NO counteracts vasoconstrictive agents such as angiotensin II (AII) and endothelin-1 (ET-1). Furthermore, NO inhibits platelet aggregation, leukocyte adhesion, smooth muscle cell proliferation, and oxidation of low-density lipoproteins (LDL) [11].In contrast to NO, endothelin-1 (ET-1) is a potent vasoconstrictor synthesized in the body. ET-1 is a bicyclic peptide consisting of 21 amino acids [12,13,14]. It plays a significant regulatory role in endothelial function. ET-1 production is stimulated by pathological conditions such as hypoxia, ischemia, hemodynamic overload, acid-base disturbances, hyperglycemia, hypercholesterolemia, and oxidative stress. Its synthesis is induced by vasoconstrictors, growth factors, cytokines, thrombin, and adhesion molecules, and is suppressed by prostacyclin, estrogens, atrial natriuretic peptide, and NO [13,15].Endothelial dysfunction is considered a key mechanism in the development of hypertension and its complications, as well as a marker of disease progression [16]. Research shows that endothelial involvement occurs in the early stages of HTN [17]. In patients with initial stages of hypertension, plasma levels of ET-1 are significantly higher than in healthy individuals, and these levels increase further in stages II and III of the disease [18]. Elevated ET-1 levels are observed not only in HTN but also in various pathological conditions [16,19,20]. The highest plasma ET-1 concentrations are reported in patients with arterial hypertension combined with atherosclerosis and in those who have experienced stroke or transient ischemic attacks [21]. During acute myocardial ischemia, ET-1 levels increase even more. Moreover, elevated ET-1 has also been reported in patients with chronic heart failure (CHF) [22,23]. Determining plasma ET-1 concentration may serve as a useful screening tool for CHF diagnosis, risk assessment, and prognosis [24].Studies have shown that adhesion molecules such as VCAM-1, ICAM-1, E-selectin, and P-selectin play a role in the development of hypertension [25,26]. Elevated blood levels of these molecules are often associated with more severe disease and treatment resistance. However, the role of E-selectin remains unclear, as its elevation may indicate both therapy resistance and therapeutic success when combined with normalization of other adhesion markers [25,26].Hemostatic markers of endothelial dysfunction, such as von Willebrand factor and thrombomodulin, are also involved in hypertension, with elevated levels frequently observed in untreated patients [27]. Angiogenesis, regulated by vascular endothelial growth factor (VEGF) and fibroblast growth factor, plays a role in the pathogenesis of endothelial dysfunction in hypertension [28]. Interestingly, VEGF suppression during anti-VEGF therapy worsens cardiovascular outcomes in hypertensive patients, suggesting a protective role of VEGF. In some cases, hypertension is considered a biomarker of anti-VEGF therapy effectiveness [29,30,31].Essential hypertension is characterized by complex endothelial dysfunctions that influence disease progression and prognosis.Type 2 diabetes mellitus (T2DM) is commonly accompanied by endothelial dysfunction, contributing to vascular complications and organ damage. This dysfunction, driven by blood glucose fluctuations and hyperinsulinemia, initiates cardiovascular disease in T2DM [32,33]. In diabetes, production of vasodilators like nitric oxide and prostacyclin decreases, while vasoconstrictors such as endothelin-1 increase. Elevated levels of adhesion molecules and pro-thrombotic factors are also observed. Key mechanisms include activation of certain enzymes and accumulation of advanced glycation end-products [33,34,35].Importantly, endothelial dysfunction can precede the clinical onset of T2DM [36]. It also contributes to complications such as diabetic retinopathy [37] and neuropathy [38], in which VEGF plays a significant role [39]. The impact of endothelial dysfunction on diabetes-related brain damage remains underexplored. There is evidence linking endothelial dysfunction biomarkers with neuronal injury, independent of diabetes duration or glycemic control, indicating that endothelial dysfunction may underlie diabetic neurodegeneration [31].Atherosclerosis is a vascular disease in which endothelial dysfunction is a key mechanism [41]. Nitric oxide (NO) deficiency plays a central role in atherosclerotic progression [41]. Statins, commonly used to lower cholesterol, also improve NO bioavailability, contributing to their anti-atherosclerotic effect [42]. Increased coagulation factor activity—such as von Willebrand factor (vWF), tissue factor (TF), and plasminogen activator inhibitor-1 (PAI-1)—is seen particularly within atherosclerotic plaques [43,44,45].VCAM-1 facilitates the adhesion of mononuclear cells and lymphocytes to the endothelium and is upregulated in areas prone to atherosclerosis. Its expression correlates with disease severity [41]. ICAM-1 and E-selectin are also overexpressed in atherosclerosis, reflecting the endothelium's heightened capacity to attract immune cells [46,47].VEGF-driven neovascularization within plaques promotes growth, instability, and thromboembolic risk [48].Despite advances in managing modifiable cerebrovascular disease (CVD) risk factors—hypertension, T2DM, smoking—the role of genetic predisposition remains crucial and actively studied. Interactions between genetic variants and environmental exposures may shape individual CVD risk. Identifying genetic markers predictive of CVD is an important goal in modern medicine.One gene of interest is EDN1, which encodes endothelin-1 (ET-1), a potent vasoconstrictor peptide. The Lys197Asn polymorphism (rs5370) in EDN1 has emerged as a potential CVD risk marker.ET-1 is a 21-amino-acid peptide synthesized primarily by endothelial cells lining blood vessels. It is one of the most potent vasoconstrictors known, contributing to elevated blood pressure. ET-1 also promotes vascular smooth muscle cell proliferation, fibrosis, apoptosis, and inflammation—processes central to atherosclerosis, myocardial hypertrophy, and vascular remodeling. Elevated plasma ET-1 levels have been observed in patients with hypertension, ischemic heart disease, and CVDs, suggesting its role in pathogenesis.The EDN1 gene, located on chromosome 6p24.1, encodes preproendothelin-1, a precursor to ET-1. Several polymorphisms in this gene may influence mRNA expression, stability, or protein structure, thereby altering ET-1 levels or activity. The Lys197Asn polymorphism results from an A-to-T substitution in codon 198, replacing lysine (Lys) with asparagine (Asn) at position 197 (rs5370).Studies examining the association between Lys197Asn and CVD or hypertension—a major CVD risk factor—have yielded mixed results. Some, especially in Asian populations, report a link between the Asn197 allele (or Asn/Asn genotype) and increased risk of hypertension or elevated blood pressure, potentially via enhanced ET-1 activity. However, studies in European populations have failed to confirm these findings or reported inverse associations. These discrepancies may stem from differences in populations, research methods, and hypertension diagnostic criteria.Objective of the Study: To analyze the prognostic significance of the Lys197Asn polymorphism in the EDN1 gene in relation to the risk of developing cerebrovascular diseases (CVDs).
2. Materials and Methods
- A total of 176 patients with CVDs aged between 30 and 75 years, who were not related and were comparable in terms of socioeconomic and ethnic status, were examined. Inclusion in the study was carried out after obtaining informed consent from each participant. The study protocol was approved by the local ethics committee (Ministry of Health of the Republic of Uzbekistan – Republican Scientific Medical Institute, Institute of Hematology, TMA, TashPMI).Depending on the severity of clinical and morphological manifestations of CVDs, the patients were divided into 3 groups:1. 37 patients with a confirmed diagnosis of stroke;2. 139 patients with pre-stroke CVDs;3. A control group consisting of a population sample of 101 residents of Uzbekistan with no hereditary predisposition, risk factors, or clinical manifestations of CVDs.The examination of the control group included: blood pressure measurement, anthropometry (height, weight), socio-demographic characteristics, smoking and alcohol consumption assessment (frequency and typical dose), physical activity level, and lipid profile analysis (total cholesterol [TC], triglycerides [TG], low-density lipoprotein [LDL], and high-density lipoprotein [HDL]).Gene expression analysis of EDN1 in peripheral blood was conducted in 88 patients.Exclusion criteria included severe comorbidities, autoimmune diseases, diagnosed tumors, psychiatric disorders, and refusal to undergo genetic testing. Each patient had a specially developed clinical case form completed.DNA was extracted from peripheral blood using the “AmpliPrime RIBO-prep” kit (“AmpliSens”, Russia), following the manufacturer's recommendations. The concentration of genomic DNA was measured using a NanoDrop 2000 device (NanoDrop Technologies, USA) at a wavelength of A260/280 nm.Genotyping of Lys197Asn Polymorphism in the EDN1 Gene (rs5370): The Lys197Asn polymorphism of the EDN1 gene (provided by NP Syntol, Moscow) was genotyped using RT-PCR on a Rotor-Gene Q thermal cycler (QIAGEN, Germany).Statistical Analysis: Association analysis of the Lys197Asn polymorphism in the EDN1 gene was conducted using a case-control model (comparison of two samples). Deviations from Hardy–Weinberg equilibrium proportions were assessed using the “GenePop” tool (“Genetics of Population”), available online (http://wbiomed.curtin.edu.au/genepop). The “OpenEpi 2009, Version 2.3” statistical software package was used to process the results.Results of the Study: The distribution of Lys and Asn alleles of the EDN1 gene and the genotypes of the Lys and Asn alleles among patients with CVDs and the control group are presented in Figures 1 and 2. The following findings were observed from the figures:
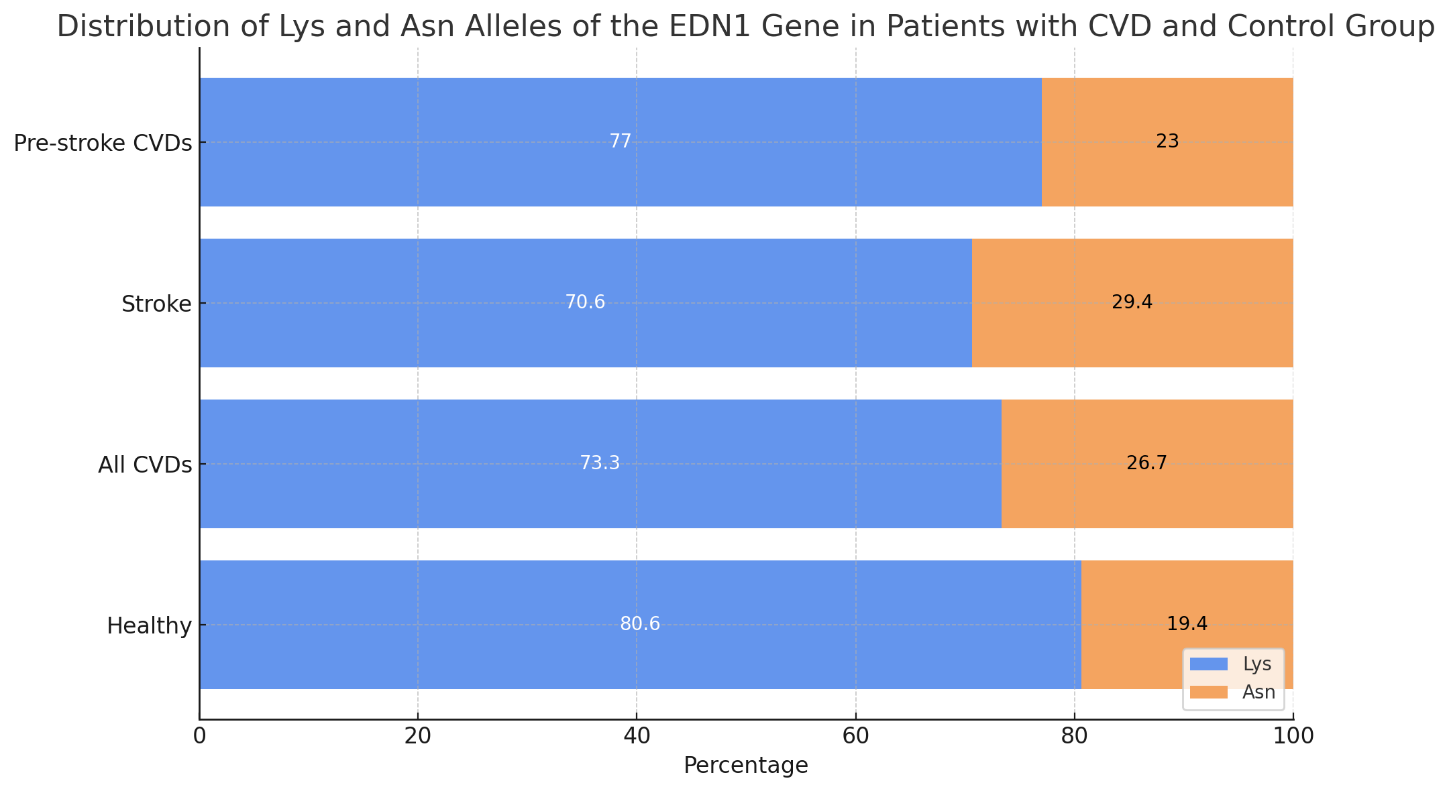 | Figure 1. Distribution of Lys and Asn Alleles of the EDN1 Gene in Patients with CVD and Control Group |
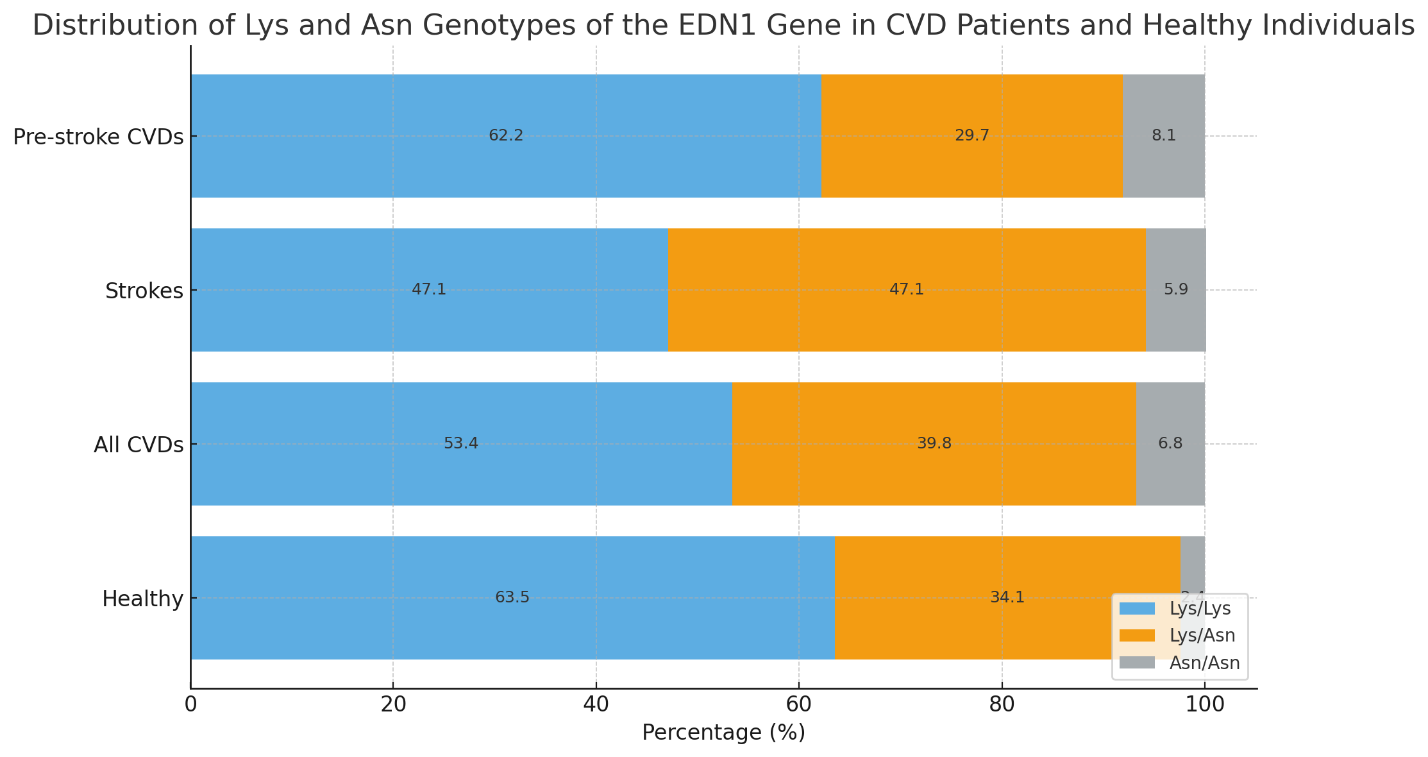 | Figure 2. Distribution of Lys and Asn Genotypes of the EDN1 Gene in CVD Patients and Healthy Individuals |
|
3. Discussion
- If the Lys197Asn polymorphism is indeed associated with the development of arterial hypertension, it is logical to assume its indirect influence on the risk of cerebrovascular diseases. However, direct studies on the association between this polymorphism and stroke or transient ischemic attacks have also produced inconsistent results. Some studies observed a higher frequency of the Asn/Asn genotype or the Asn allele in patients with ischemic stroke compared to healthy controls, particularly in patients with specific stroke subtypes (e.g., cardioembolic) or in combination with other risk factors (e.g., hypertension). Other studies have not found a statistically significant association between the Lys197Asn polymorphism and the risk of any type of stroke or ischemic stroke in particular. There are also reports of a possible association of this polymorphism with hemorrhagic stroke, suggesting a potential role of ET-1 in weakening the vascular wall, although these findings are also inconclusive.Despite the contradictory data, several mechanisms can be proposed through which the Lys197Asn polymorphism might influence CVD risk: alteration of ET-1 protein function due to the amino acid substitution may affect the stability, activity, or interaction of ET-1 with its receptors, modifying its vasoconstrictive effect or its ability to stimulate cell proliferation. Enhanced vasoconstrictive action of ET-1 may promote the development and progression of hypertension, a strong risk factor for ischemic stroke. ET-1 contributes to inflammation, proliferation of smooth muscle cells, and fibrosis, promoting the formation and instability of atherosclerotic plaques, which may lead to embolism and ischemic stroke. The effect of the polymorphism may vary depending on the type of stroke (ischemic/hemorrhagic), its subtype (atherothrombotic, cardioembolic, etc.), and the presence of comorbid conditions such as hypertension and diabetes.Loss of information due to genotype grouping may also play a role. Analysis based on genotype groups (Lys/Lys, Lys/Asn, Asn/Asn) may miss subtle associations that could be revealed by allele-level analysis or by using dominant/recessive models.At this point, due to the inconsistent findings, the Lys197Asn polymorphism in the EDN1 gene cannot be recommended as a reliable independent risk marker for screening or individualized preventive strategies for cerebrovascular diseases in clinical practice. The Lys197Asn (rs5370) polymorphism in the EDN1 gene is a genetic variation that could theoretically influence the risk of cerebrovascular diseases through modulation of endothelin-1, a key regulator of vascular tone and structure. Despite some preliminary data suggesting a possible association with arterial hypertension and stroke, the findings remain contradictory. Further large-scale, multicenter, and well-designed studies are needed, taking into account population heterogeneity, gene-gene and gene-environment interactions, and detailed clinical characterization of patients to fully assess the role of this polymorphism in the pathogenesis and risk of cerebrovascular diseases. Current data do not support its use as a clinical marker, but ongoing research in this area continues to contribute to understanding the complex genetic basis of these serious conditions.
4. Conclusions
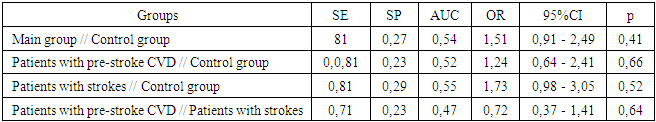
- The Lys197Asn polymorphism in the EDN1 gene may be associated with an increased risk of cerebrovascular diseases, particularly in the presence of the Asn allele and the Asn/Asn genotype. Sensitivity (SE) values across all groups were relatively high (0.71–0.81), indicating that the Lys197Asn polymorphism is frequently observed in patients with CVD. However, low specificity (SP) values (0.23–0.29) suggest that the polymorphism also occurs frequently in healthy individuals, limiting its prognostic value. Further studies investigating the interaction between genetic and environmental factors, as well as the ethnic background of patients, may help in developing individualized prevention and treatment strategies.
ACKNOWLEDGEMENTS
- The authors express their sincere gratitude to all participants for their voluntary involvement and willingness to provide biological samples, as well as to the medical personnel for their assistance in material collection. The study was conducted using the authors' own financial resources. Special thanks are extended to the team of the Republican Specialized Scientific-Practical Medical Center of Hematology of the Ministry of Health of the Republic of Uzbekistan and its leading expert, Doctor of Biological Sciences, Professor K.T. Boboev, for assistance in organizing the research. We also thank Doctor of Medical Sciences, Professor Z.I. Adambaev of Urgench State Medical Institute for his contribution to writing the article, and Doctor of Medical Sciences, Professor Y.N. Madjidova of Tashkent State Medical University for general supervision of the study. The authors further thank Candidate of Medical Sciences, Associate Professor S.O. Inoyatova of Tashkent State Medical University and E.I. Abdukadirov for their leadership in data collection and project funding.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML