-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(8): 2558-2561
doi:10.5923/j.ajmms.20251508.29
Received: Jun. 28, 2025; Accepted: Jul. 20, 2025; Published: Aug. 6, 2025

Sudden Cardiac Death: Possibilities of Forensic Diagnostics
Turonov B. S.1, Ruziev Sh. I.2
1Scientific and Practical Center of Judicial Medical Expertise of the Republic, Uzbekistan
2Tashkent State Medical University, Uzbekistan
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The article analyzes modern visualization methods and the importance of morphological changes in sudden cardiac death, due to the high frequency of fatal outcomes occurring within a short period of time after the onset of the first symptoms or without them at all. The greatest difficulties in forensic practice arise when establishing the cause of death in cases of the absence of macroscopically expressed changes. Modern approaches to forensic diagnostics of SCD include a complex of morphological, histological, immunohistochemical studies. Of particular importance is the detection of signs of ischemic myocardial damage, ventricular fibrillation, cardiomyopathy and channelopathies. The development and implementation of algorithms for postmortem diagnostics can improve the accuracy of establishing the cause of death, especially in young people.
Keywords: Sudden cardiac death, Forensic medical examination, Myocardial infarction, Cardiomyopathy, Morphology
Cite this paper: Turonov B. S., Ruziev Sh. I., Sudden Cardiac Death: Possibilities of Forensic Diagnostics, American Journal of Medicine and Medical Sciences, Vol. 15 No. 8, 2025, pp. 2558-2561. doi: 10.5923/j.ajmms.20251508.29.
Article Outline
1. Introduction
- Sudden cardiac death (SCD) remains one of the leading causes of mortality, especially among young people. Modern research focuses on the molecular genetic aspects of SCD, which opens up new prospects in forensic diagnostics. According to a review published in the journal "Forensic Medical Examination", molecular genetic studies can identify mutations in genes associated with an increased risk of SCD [1,2,3,4]. These genes include those that encode ion channels and proteins involved in the regulation of heart rhythm. Identification of these mutations contributes to a more accurate diagnosis of the causes of SCD and can be used in forensic practice to establish a genetic predisposition to fatal arrhythmias. In economically developed countries, SCD ranges from 18.6 to 128 cases per 100,000 inhabitants per year. Epidemiological studies show that ischemic heart disease predominates in the structure of SCD. In individuals under 45 years of age, SCD is most often caused by the presence of various forms of cardiomyopathy. C. Andersson and R. Vasan [5,6] when analyzing the death of individuals under 35 years of age most often and in equal percentages noted cases of sudden death caused by the presence of ischemic heart disease or various forms of cardiomyopathy. In 40% of posthumously established diagnoses, sudden death of individuals with cardiomyopathy was associated with mutations in the genes responsible for the structure of the heart, in 60% - with mutations in genes related to electrolyte metabolism disorders - the functioning of cardiomyocyte channels [7,8,9]. It should be noted that impaired conduction of the heart muscle can be caused by mutations in the genes responsible for the synthesis of proteins in desmosomes [10,11]. According to statistics from the World Health Organization, 17.3 million people died from CVD in 2008, accounting for 30% of all deaths worldwide. Of this number, 7.3 million people died from ischemic heart disease and 6.2 million people from stroke. According to WHO forecasts, by 2030 about 23.6 million people will die from CVD, mainly from heart disease and stroke, which will likely retain a confident lead among the main causes of death. In forensic practice, the main criteria for establishing the cause of death of those who died suddenly are the indicators of macro and microscopic studies aimed at identifying morphological changes in the affected organs and tissues of the corpse. [12,13,14,15,16]. Further study of the clinical, morphological and molecular biological features of different forms of SCD is required, which will allow us to understand the mechanisms of its development and provide the key to developing effective methods for diagnosing, preventing and treating the disease. Modern forensic medical examination actively uses methods of laboratory and instrumental diagnostics, but there are still a number of unresolved problems that require further research.
2. Research Aim
- Study and improve data on the pathomorphological mechanisms of sudden cardiac death and the importance of determining thanatogenesis in forensic medical examination.
3. Materials and Methods
- Clinical-anamnesis, morphological and statistical examinations were conducted. As material for the study, 126 cases were taken from the autopsy examination at the Republican Scientific and Practical Center of Forensic Medicine during 2021-2025, in which the diagnosis of TYU was confirmed based on clinical and morphological data. During the autopsy, samples were taken from all internal organs, including the heart, for histological examination. The sections were processed in the usual way, paraffin blocks were prepared, histological sections were prepared from them and stained with hematoxylin and eosin. Histological sections prepared from all internal organs were examined in detail under 10, 20, 40 objects of a light microscope, and areas rich in information from the heart, lung, kidney tissue and blood vessels, as well as places where pathomorphological changes were observed, were photographed.
4. Results and Discussion
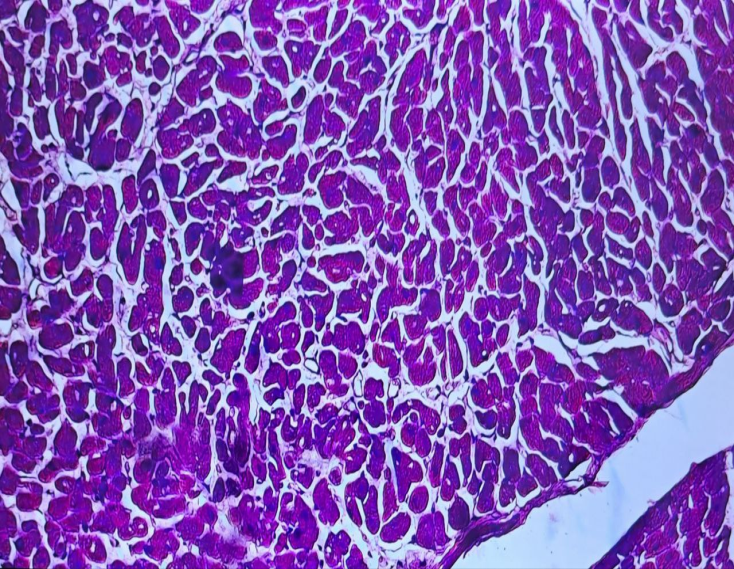
- According to the results of forensic histological analysis, the following pathomorphological changes were detected in the heart muscle tissue (Fig. 1) against the background of sudden death. Signs of dystrophy were noted in cardiomyocytes in the heart muscle tissue in the form of granular and segmented cells. Loss of signs of transverse dilatation, lysis of myofibrils, and dispersion of the cytoskeleton were observed in the cell cytoplasm. Such dystrophic changes develop against the background of ischemia, hypoxia, and stress effects and lead to a complete loss of cardiomyocyte function. Fragmentation, i.e. cell fragmentation, cytoplasmolysis were detected in some cells. This indicates terminal cell damage. At the same time, a disproportion in cell size was observed, pathological hypertrophy in some cardiomyocytes, and atrophy in others. This condition occurs as a result of focal hemodynamic imbalance in the heart muscle tissue. Interstitial edema of the heart tissue is directly related to the state of hypoxia and venous stasis of the lesion. The edema weakens the stromal structure, disperses the parenchymal tissues, and disrupts the biochemical connections between cells. Inadequate blood filling and microcirculatory changes are observed in the blood vessels of the myocardium, i.e., dilation of the microcirculatory tract, especially capillaries and venules. Stasis of erythrocytes in the blood vessels, i.e., cessation or significant slowing of blood flow, is noted. These changes are characteristic of sudden death without resuscitation efforts, causing TCU in a state of cardiac decompensation. Such changes often occur as a result of cardiomyopathies and neurogenic mechanical death.
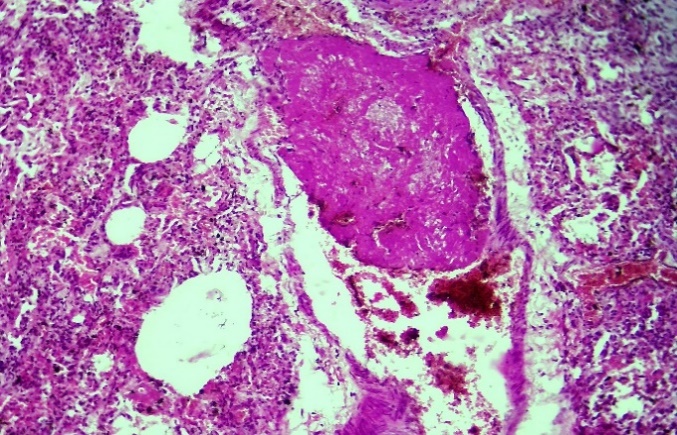 | Figure 2. Sudden death, fibrin thrombus formation in the large branch of the pulmonary artery 2 weeks after delivery, fibrin thrombus in the capillaries H&E staining. Magnification 10x10 |
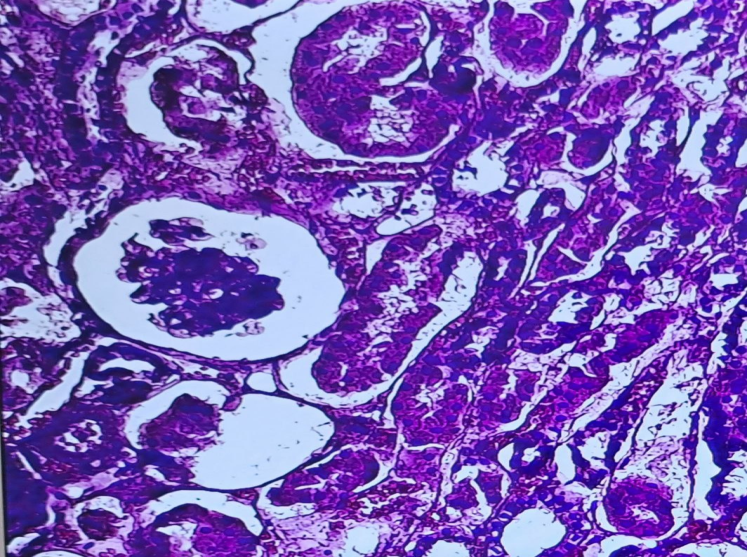 | Figure 3. Convoluted tubule necrosis atrophy in the glomerular capillary network in sudden death.H&E staining. Magnification 10x10 |
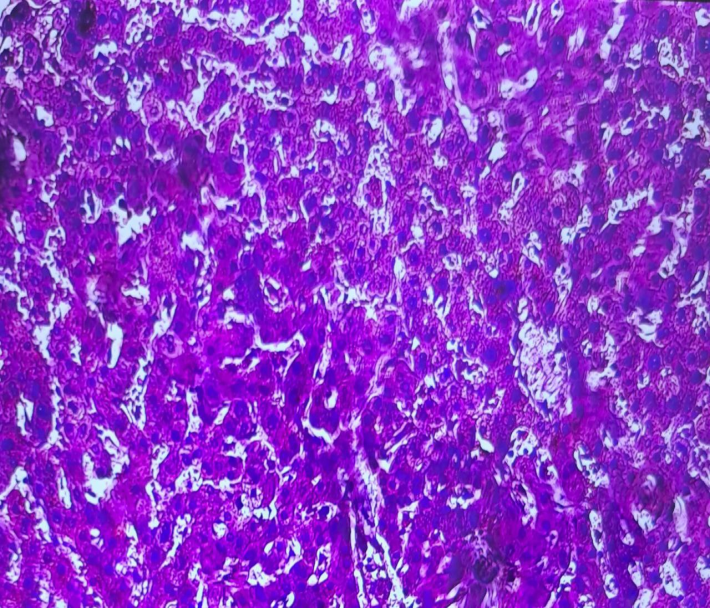 | Figure 4. Hydropic and vacuolar dystrophy in liver hepatocytes, caused by acute venous insufficiency. H&E staining. Magnification 10x10 |
5. Conclusions
- We have established that in those who died from sudden cardiac death, there are characteristic pathomorphological macro and microscopic changes in the internal organs, which are most often found in this syndrome. Sudden cardiac death (SCD) is one of the main causes of death among the working-age population and poses a serious diagnostic problem in forensic practice. In such cases, a comprehensive approach is required to determine the cause of death, including morphological, histological, and immunohistochemical methods. Today, the use of modern technologies such as molecular autopsy and visualization of subcellular changes expands the capabilities of pathogenetic analysis and allows detecting latent hereditary cardiopathies and channelopathies in which macroscopic changes are not observed. Standardization of forensic diagnostic algorithms, i.e., complete collection of anamnesis, analysis of clinical-pathological correspondence, and integration of laboratory methods, is of great importance. In young patients and in cases where atherosclerosis of the coronary arteries has not been detected, the main attention should be paid to the detection of cardiomyopathies and primary heart rhythm disorders. This requires collaboration between forensic experts, geneticists, and pathologists. In conclusion, improving forensic diagnostic methods based on modern and proven medical knowledge in cases of sudden cardiac death will not only help determine the exact cause of death, but also prevent relapses in families with a hereditary predisposition.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML