-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(8): 2552-2557
doi:10.5923/j.ajmms.20251508.28
Received: Jun. 29, 2025; Accepted: Jul. 23, 2025; Published: Aug. 6, 2025

Immunogistochemical Study of Morphological Changes in Diffuse Forms of Breast Cancer
Yigitaliyev A. B.1, Gafur-Akhunov M. A.2, Tursunov X. Z.3
1Fergana Medical Institute of Public Health, Fergana, Uzbekistan
2Center for the Development of Professional Qualifications of Medical Workers, Tashkent, Uzbekistan
3Tashkent State Medical University, Tashkent, Uzbekistan
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This article presents the results of a comparative immunohistochemical study of diffuse and nodular forms of breast cancer, based on the expression of key molecular markers such as Ki-67, p53, Bcl-2, CD4, and CD34. The study included 83 patients treated in the regional oncology centers of Uzbekistan, with detailed morphological and immunological evaluation of tumor tissues. Diffuse forms of breast cancer demonstrated higher proliferative activity, greater genetic instability, and increased angiogenesis, as evidenced by elevated levels of Ki-67, p53, and CD34 expression, along with lower CD4 immune cell infiltration. In contrast, nodular forms showed a more balanced immunological response and lower tumor aggressiveness. The findings confirm the diagnostic and prognostic significance of immunohistochemical profiling in guiding treatment decisions, especially in biologically aggressive diffuse breast cancer. The study supports the use of molecular markers to personalize therapeutic approaches and improve patient outcomes.
Keywords: Diffuse breast cancer, Ki-67, p53, Bcl-2, CD4, CD34, Immunohistochemistry, Tumor microenvironment, Angiogenesis, Prognostic markers, Breast cancer subtypes, Targeted therapy
Cite this paper: Yigitaliyev A. B., Gafur-Akhunov M. A., Tursunov X. Z., Immunogistochemical Study of Morphological Changes in Diffuse Forms of Breast Cancer, American Journal of Medicine and Medical Sciences, Vol. 15 No. 8, 2025, pp. 2552-2557. doi: 10.5923/j.ajmms.20251508.28.
Article Outline
1. Introduction
- Breast cancer (breast cancer) is the most common oncological disease among women, with approximately 2.3 million new cases detected worldwide annually, and this disease accounts for 24.5% of cancer cases in women [4]. Approximately 685,000 women die each year from breast cancer.The number of registered breast cancer cases in the Republic of Uzbekistan for the first time in 2019-2021 was 10,984 people, with an average morbidity rate of 11.0 per 100,000 population. Of these: in 2019 - 3718 patients, the incidence rate - 11.2 per 100 thousand people, in 2020 - 3317 patients, the incidence rate - 9.8 per 100 thousand people, in 2021 - 3949 patients, the incidence rate of breast cancer with stages I-II per 10 thousand people in 2019 and 2020 was 51.8%. - 63.0%, in 2021 - 62%. At the same time, despite the existing visualization of the organ, the proportion of patients with stage III-IV breast cancer remains high: in 2019 - 32.9%, in 2020 - 34.4%, in 2021 - 32.8%. From 2019 to 2021, the mortality rate averaged 5.2 per 100,000 population. In 2019, 2020, 2021, the 5-year survival rate for breast cancer was 45.4%, 45.1%, and 45.1%, respectively [1,2,3].Among all clinical forms of breast cancer, diffuse forms are one of the most important clinical problems. It accounts for 2-15% of all clinical manifestations of breast cancer. Because diffuse breast cancer grows without forming a distinct nodule, it is difficult to detect at an early stage. This affects the treatment and prognosis of the disease. The diffuse form of breast cancer is less studied than other forms, and its accurate diagnosis and treatment strategies remain an urgent problem.Diffuse forms of this disease are characterized by a clinically severe course, rapid metastasis, and a low response to treatment. Therefore, the problem of studying biomarkers influencing the early detection and prognosis of diffuse forms of breast cancer is becoming increasingly relevant [1,2].In recent years, the integration of immunohistochemical analyses into morphological research methods has opened up new possibilities for the analysis of oncological diseases. In particular, Ki-67, p53, and Bcl-2 markers are of great importance in assessing cell proliferation, apoptosis, and the molecular properties of the tumor in breast cancer. If Ki-67 determines the degree of proliferation of tumor cells, then the expression of the p53 gene in the mutant form is associated with high tumor aggressiveness and prognosis. Also, Bcl-2 is an important marker that determines the degree of tumor resistance to therapy with its ability to inhibit apoptosis [3,4].In addition, the condition of the tumor microenvironment - that is, the state of the immune cells and vascular contents surrounding the tumor - also has a direct influence on the clinical prognosis. From this point of view, the study of CD4 (helper T-cells) and CD34 (angiogenesis-associated endothelial marker) plays an important role in revealing the immunological features of breast cancer [5,6].Ki-67-protein (also known as MKI 67) is a cellular marker for proliferation and may be used in immunohistochemistry. This is closely related to cell proliferation. In interphase, the Ki-67 antigen can only be detected in the cell nucleus, while in mitosis, most of the protein is transferred to the chromosome surface. Ki-67 protein is present in all active phases of the cell cycle, but not in resting cells. The cellular composition of the Ki-67 protein increases significantly during the cell progression through the C phase of the cell cycle. In breast cancer, Ki 67 identifies a high proliferative proportion of E-R-positive breast cancer patients who benefit more from assisted chemotherapy. Ki-67 is an excellent marker for determining the growth portion of a particular cell population. The proportion of Ki-67-positive tumor cells (Ki-67 marking index) is often associated with the clinical course of cancer. The best studied examples in this context are prostate, brain, and breast carcinomas, as well as nephroblastoma and neuroendocrine tumors. The prognostic value of survival and tumor recurrence for this type of tumor has been repeatedly proven in one-dimensional and multidimensional analyses. Ki-67-nuclear protein is a marker of proliferative activity of tumor cells and is evaluated as a percentage. Ki 67 is used for diagnostic purposes to determine the biological potential of malignant tumors in humans. The staining of nuclear cells is described as follows. <10% low activity, 10-20% moderate activity, >20% high proliferative activity. Through these results, it is possible to determine the prognostic factor of cancer.The antigen for these antibodies is the w r53 protein, which controls the course of cell cycle processes, as well as the presence of damage in the genome, which can lead to the further development of pathology. w r53-dependent apoptosis is a powerful selector, preventing the accumulation of mutations, and if they have already appeared, w r53-dependent apoptosis allows the destruction of such potentially dangerous cells for the organism. Mutations have been found in 50% of all types of cancer cases. This gene encodes a transcription factor that controls the entry of cells into the cell cycle. Many intracellular systems that monitor the "health" of the cell send signals about "malfunctions" to the w r53 protein. With its help, the cell decides whether to divide or not. If a cell is irreparably damaged, the w p53 protein triggers a chain of events that lead to cell "suicide," otherwise called apoptosis. Cells that lack or don't function properly with w p53 are unable to control such self-regulation and continue to divide even when it's dangerous for the body. Like all tumor suppressants, w p53 controls the normal course of the cell cycle. w p53 is a transcription factor that regulates the cell cycle, this reagent performs the function of suppressing the formation of malignant tumors. The w p53 gene is an anti-oncogenic agent.CD34 - membrane protein, a molecule of intercellular adhesion (intercellular adhesion) that plays a role in the early stages of hematopoiesis. CD34 mediates the attachment of stem cells to the extracellular matrix of bone marrow or directly to stromal cells. A protein serves as a barrier for attaching specific glycans, allowing root cells to attach to lectins produced by stromal cells or other components of the bone marrow. In addition, highly glycosylated CD34 provides carbohydrate ligands for selectins.In molecular biology, CD4 (differentiation cluster 4) is a glycoprotein that serves as a coreceptor for t-cell receptors. CD4 helper cells are located on the surface of immune cells such as monocytes, macrophages, and dendritic cells. In humans, the CD4 protein is encoded by the CD4 gene. CD4 helper cells are white blood cells that are an integral part of the human immune system. They are often called CD4 cells, t helper cells, or T4 cells. They are called helper cells because one of their main functions is to spread to other types of immune cells, including CD8 killer cells, which subsequently destroy infectious particles. If the number of CD4 cells decreases, for example, after untreated HIV infection or suppression of immunity before transplantation, the body becomes vulnerable to various infections that can be fought in other ways.Bcl 2 - suppresses apoptosis in multicellular systems, including lymphohematopoietic and neuronal cells. It regulates cell death by controlling the permeability of the mitochondrial membrane. Preventing the release of cytochrome c from the mitochondria inhibits phosphatases by binding an apoptosis-activating factor.This article is used in the formation of diagnostic and prognostic approaches based on the study of the expression of the above immunohistochemical markers and morphofunctional changes in the tumor microenvironment in diffuse breast cancer.
2. Materials and Methods of Research
- The main material of the presented methodological recommendation was the results of a comprehensive examination and treatment of 83 patients diagnosed with diffuse forms (63 patients) and nodular forms (20 patients) of breast cancer in the Fergana and Tashkent regional branches of the Republician oncology center. For a comprehensive diagnosis, all patients underwent clinical, laboratory, and instrumental studies (X-ray, ultrasound, MSCT, MRI, PET/CT), as well as pathomorphological and immunohistochemical studies.For pathomorphological examination, cytological, histological, and immunohistochemical studies were conducted. At the same time, the histogenetic affiliation of the tumor and the degree of malignancy of malignant tumors were determined. During the immunohistochemical study, the indicators of such molecular genetic markers as Ki-67, Bcl-2, p53 were studied.In addition, immunohistochemical studies and determination of the number and activity of lymphocytes (CD4 and CD34) in the area of peritumor and tumor processes were carried out.All 83 patients underwent pathomorphological examination (cytological, histological), and a total of 40 patients with diffuse and nodular forms of breast cancer were divided into 2 groups with histological materials and immunohistochemical examination.When analyzing the nature of the clinical course of diffuse forms of breast cancer, it was established that out of 63 patients, 5 (7.9%) had the mastic form of breast cancer, 48 (76.2%) had the edematous-infiltrative form, 7 (11.1%) had the panser form, and 3 (4.8%) had the clinical form of Paget's cancer.All patients with cervical cancer included in the study were women, whose age ranged from 33 to 78 years, and the average age was 53.4 ± 1.3 years.The duration of the medical history, depending on the nature of the lesion, ranged from 1 to 13 months.In the immunohistochemical study, we analyzed the molecular structures of cells, the location of cells, histogenesis of tumor cells, the proliferative and mitotic activity of cells (Ki-67), the expression of the suppressor gene (p53) and the apoptosis index in tumor cells (Bc1-2), as well as the state of CD4 (helper T-cells) and CD34 (angiogenesis-associated endothelial marker) in the tumor microenvironment.Immunohistochemical studies were conducted in the pathomorphology laboratory of the RSNPMC of the Republic of Uzbekistan. The sections were taken from wet archives after surgical removal of tumors. The materials were processed on a "Thermo Fisher Scientific" histological processor for 12 hours for histological examination of biopsy and surgical materials according to the instructions. Pieces with a thickness of 4 μm were prepared from kerosene blocks, stained with hematoxylin-eosin, and subjected to microscopic examination under the microscope "Leica Microsystems," Germany. The stages of the immunohistochemical study are summarized in Table 1.
|
3. Research Results and Discussions
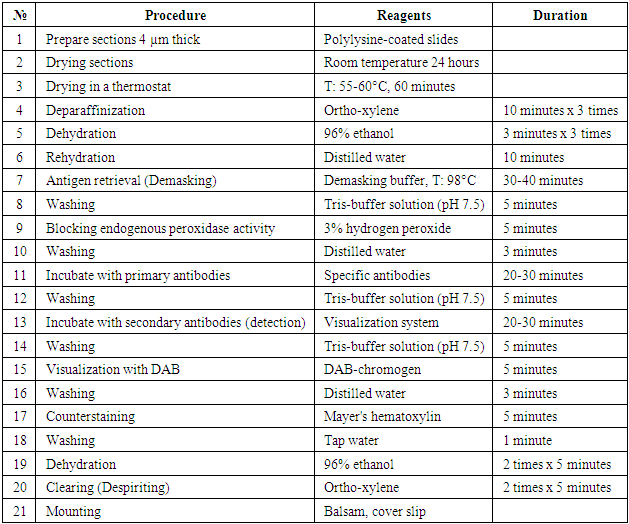
- Diffuse forms of breast cancer Ki-67-reagent is a cellular marker for proliferative activity. In breast cancer, Ki-67 is used for diagnostic purposes to determine the biological potential of malignant tumors in humans. The staining of nuclear cells is described as follows. <10% low activity, 10-20% moderate activity, >20% high proliferative activity. Based on these results, it is possible to determine the prognostic factor of cancer. 20 patients were selected for this study. The obtained results showed that out of 20 patients, 12 (60%) had a high degree of positive reaction, 6 (30%) had a medium degree of positive reaction, and 2 (10%) had a low positive reaction.Microscopic appearance: infiltrative character in the tubular epithelium of the hyperplastic breast: polymorphic cells, hyperchromatic nuclei, multiple pathological mitoses, foci of intravascular invasion in some places. Malignant tumor cell nuclei are stained dark brown.
 | Figure 1. High-degree positive reaction of the Ki67 reagent in diffuse forms of breast cancer. IGX - Dab chromogen. Ob10xok40 |
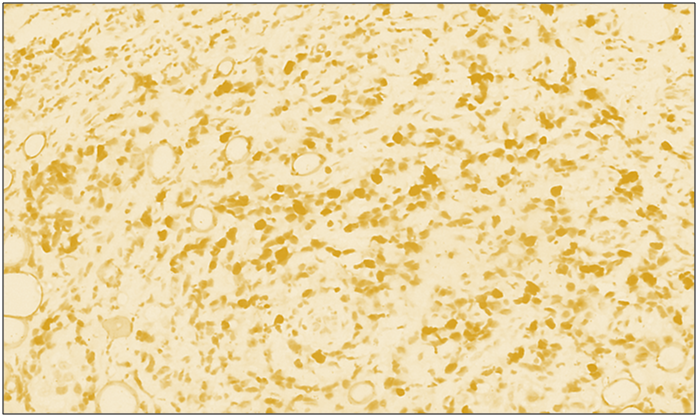
 | Figure 2. High positive reaction of reagent p53 in diffuse forms of breast cancer. IGX - Dab chromogen. Ob10xok40 |
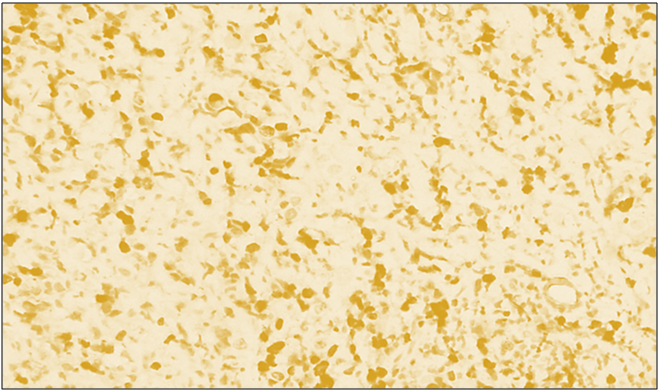
 | Figure 3. High proliferative positive reaction of the Bcl-2 marker in diffuse forms of breast cancer. IGX - Dab chromogen. Ob10. Ok40 |
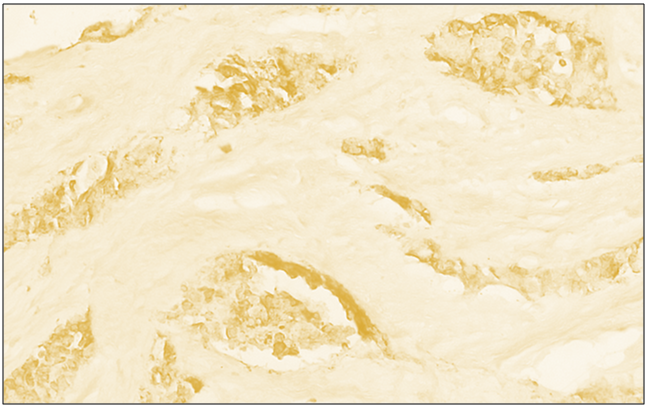
 | Figure 4. Low positive reaction of the CD4 reagent in diffuse forms of breast cancer. IGX - Dab chromogen. Ob10xok40 |
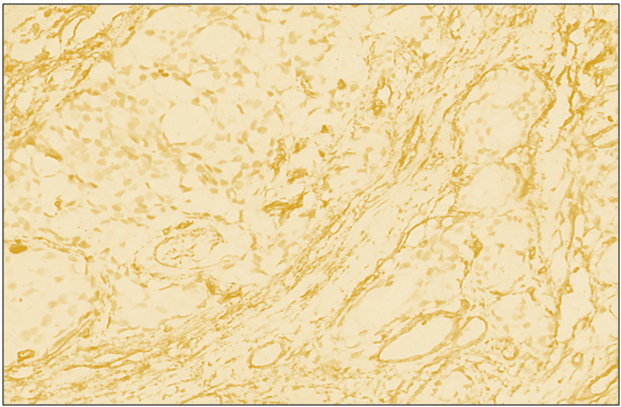 | Figure 5. Positive reaction of reagent CD34 in diffuse forms of breast cancer. IGX - Dab chromogen. Ob10. Ok40 |
4. Conclusions
- Analysis of the immunohistochemical study showed that in diffuse forms of breast cancer, there is a certain profile for the degree of malignancy and tumor markers, the goal of which is mainly to determine the aggressiveness of the tumor process. This should be taken into account when determining treatment tactics, choosing chemotherapy, targeted treatment, and determining the prognosis of the disease. Immunohistochemical markers CD4 (helper T-cells) and CD34 (angiogenesis-associated endothelial marker) in the studied tumor microenvironment, each of which has specific features in determining tumor aggressiveness and can be recommended as a prognostic factor in diffuse forms of breast cancer.The economic efficiency of the method has been proven and significantly reduces the cost of treatment of diffuse forms of breast cancer.The study of quantitative indicators of the immune system in diffuse forms of breast cancer showed that the number of T-helper inductors in tumor tissue was significantly higher by 100% (by 2 times), which determined a high immunoregulatory index (2.0).Assessment of morphological and immunological criteria in patients with diffuse forms of breast cancer showed their interrelationship. Morphological study revealed large and medium-sized atypical glandular structures, large hyperchromic cells, lymphocytic and histiocytic infiltration, which made it possible to identify the observed changes in immunological parameters.The indicators of Ki-67, p53, Bcl-2 in diffuse forms of breast cancer are characterized by a higher expression of the indicators of Ki-67, p53, Bcl-2 in diffuse forms of breast cancer compared to the nodular form. In particular, the Ki-67 proliferation index is detected at a high level, which indicates the tumor's ability to grow rapidly and its aggressive clinical course. The expression of p53 in the mutated form indicates genetic instability of the tumor, i.e., disruption of tumor suppressor function and weakening of cellular apoptosis. The anti-apoptotic protein Bcl-2 participates in preserving viable cells and increasing their resistance to chemotherapy, reducing the tumor's response to treatment.Immunohistochemical analysis of these markers is important when choosing treatment tactics for diffuse breast cancer. That is, in cases of high Ki-67 and p53 expression, it is advisable to use more aggressive and high-dose combined chemotherapeutic regimens. In cases of high Bcl-2 expression, the use of targeted anti-Bcl-2 therapy may also be considered.Thus, the compatibility of morphological and molecular-genetic factors is the basis for predicting diffuse forms of breast cancer and forming an individualized therapeutic approach. Such a comprehensive approach serves to improve the quality of life and increase the patient's life expectancy in clinical practice.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML