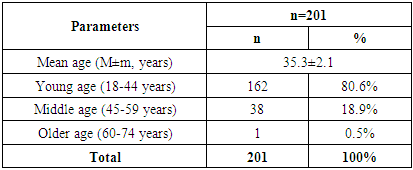-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(7): 2342-2348
doi:10.5923/j.ajmms.20251507.54
Received: Jun. 23, 2025; Accepted: Jul. 17, 2025; Published: Jul. 24, 2025

Impact of Chronic Kidney Disease and Renal Replacement Therapy on Reproductive Health and Life Quality
S. S. Kariev, F. R. Nasirov
Republican Specialized Scientific and Practical Medical Center of Urology, Tashkent, Uzbekistan
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The aim of the study was to evaluate the dynamics of erectile function in patients with chronic kidney disease over a long period of time - from the initial treatment to 12 months after hemodialysis, to analyze the impact of chronic kidney disease and hemodialysis on erectile function, as well as to identify the key pathogenetic mechanisms underlying this process. Background. Chronic kidney disease is a serious medical and social problem affecting various aspects of a patient's health, including his cardiovascular system, metabolic processes and quality of life. One of the significant but often underestimated complications of chronic kidney disease is erectile dysfunction, which has a significant impact on the psycho-emotional state and general well-being of patients. Material and methods. The study included 201 patients with chronic kidney disease who went to the Republican Specialized Scientific and Practical Medical Center of Urology with complaints of erectile dysfunction from 2022 to 2025. Results. The mean age of patients was 35,3±2,1 years. The majority of patients belonged to the young age category (18-44 years), which confirms the prevalence of CKD among the able-bodied population - 162 (80.6%). Middle age (45-59 years) was registered in 38 (18,9%) cases. Discussion. It was revealed that the severity of reproductive disorders depended not only on the stage of CKD and duration of replacement therapy, but also on individual factors such as age, presence of concomitant endocrine and cardiovascular diseases, etc. Conclusion. Reproductive disorders in patients with CKD are a common and significant problem affecting not only the quality of life but also the long-term outcome of the disease. Patients with CKD undergoing hemodialysis have been found to have significant endocrine and vascular disorders leading to reproductive disorders, including hypogonadism and erectile dysfunction.
Keywords: Chronic kidney disease, Reproductive disorders, Sexual dysfunction, Infertility, Hypogonadism, Hormonal disorders, Hemodialysis, Fertility
Cite this paper: S. S. Kariev, F. R. Nasirov, Impact of Chronic Kidney Disease and Renal Replacement Therapy on Reproductive Health and Life Quality, American Journal of Medicine and Medical Sciences, Vol. 15 No. 7, 2025, pp. 2342-2348. doi: 10.5923/j.ajmms.20251507.54.
Article Outline
1. Introduction
- Chronic kidney disease (CKD) is a serious medical and social problem affecting various aspects of a patient's health, including his cardiovascular system, metabolic processes and quality of life. One of the significant but often underestimated complications of CKD is erectile dysfunction (ED), which has a significant impact on the psycho-emotional state and general well-being of patients. Erectile dysfunction in patients with CKD is associated with a number of pathogenetic factors, including endothelial dysfunction, hormonal changes, uremic intoxication, and vascular dysfunction. Chronic kidney disease is a multifactorial progressive disease accompanied by systemic disorders of metabolism, endocrine and immune regulation. Reproductive disorders are a common complication of CKD. In the late stages of the disease, the secretion of sex hormones, spermatogenesis cycle is disturbed, which leads to erectile dysfunction, infertility and decreased libido. Despite the success of renal replacement therapy, only some patients achieve full recovery of reproductive function. The article presents current ideas about the pathogenesis, clinical manifestations and approaches to the treatment of reproductive disorders in patients with CKD. The need for a multidisciplinary approach, including nephrologist, endocrinologist and andrologist, in the management of such patients is emphasized.One of the often underestimated but clinically significant problems is reproductive dysfunction in patients. According to various authors, up to 90% of men with end-stage CKD experience some forms of reproductive disorders [1,2].Pathogenesis of reproductive disorders in CKD. Urogenital disorders are caused by a combination of the following factors:- hyperprolactinemia;- resistance to gonadotropins;- impaired gonadoliberin secretion;- decreased levels of testosterone, estradiol and progesterone;- intoxication with uremic metabolites.Hypogonadism, decreased libido, erectile dysfunction and oligozoospermia are developed in such type of patients [3].Effect of hemodialysis and peritoneal dialysis. Regular dialysis improves the general condition, but only partially restores the hormonal background. Some studies have reported a moderate increase in testosterone levels and a decrease in prolactin after conversion from peritoneal dialysis to hemodialysis [4]. However, fertility remains low. Reproductive function after kidney transplantation. Successful kidney transplantation contributes to partial or complete restoration of endocrine function of the hypothalamic-pituitary-gonadal system. Patients have improved libido and spermatogenesis [5].However, a number of factors limit the restoration of fertility:- the use of glucocorticoids and calcineurin inhibitors (cyclosporine, tacrolimus);- hypertension and hyperlipidemia;- residual uremic intoxication;- injury of pituitary gland and gonads due to prolonged CKD.Our findings may contribute to the development of more efficient management of patients with CKD, aimed at improving their reproductive health and overall quality of life.
2. Material and Methods
- In this study, we investigated 201 patients with CKD who visited to Republican Specialized Scientific and Practical Medical Center of Urology with complaints of ED from 2022 to 2025. The inclusion criteria were as follows:- Age over 18 years.- Confirmed diagnosis of CKD (stages 2-5).- Patients receiving renal replacement therapy (hemodialysis).- Presence of data on laboratory and clinical parameters in dynamics.Exclusion criteria consisted of the following factors:- Decompensated comorbidities preventing participation in the study.- Inadequate clinical and laboratory data.- Patients with terminal oncologic diseases.- Female patients.Examination of patients included:- anamnesis collection (reproductive function, libido, fertility, sexual activity);- physical examination;- laboratory tests: testosterone level, LH, FSH;- ultrasound Doppler ultrasonography of the cavernous and dorsal arteries of the penis and scrotal organs.Statistical analysis was performed using Statistica 12.0 software, using Student's t-test, χ² and correlation analysis (p < 0.05 was considered statistically significant).
3. Results
- The mean age of patients was 35,3±2,1 years. The majority of patients belonged to the young age category (18-44 years), which confirms the prevalence of CKD among the able-bodied population - 162 (80.6%). Middle age (45-59 years) was recorded in 38 (18.9%) cases. Older patients (60-74 years) accounted for the smallest proportion - only 1 (0.5%) patient (Table 1).
|
|
|
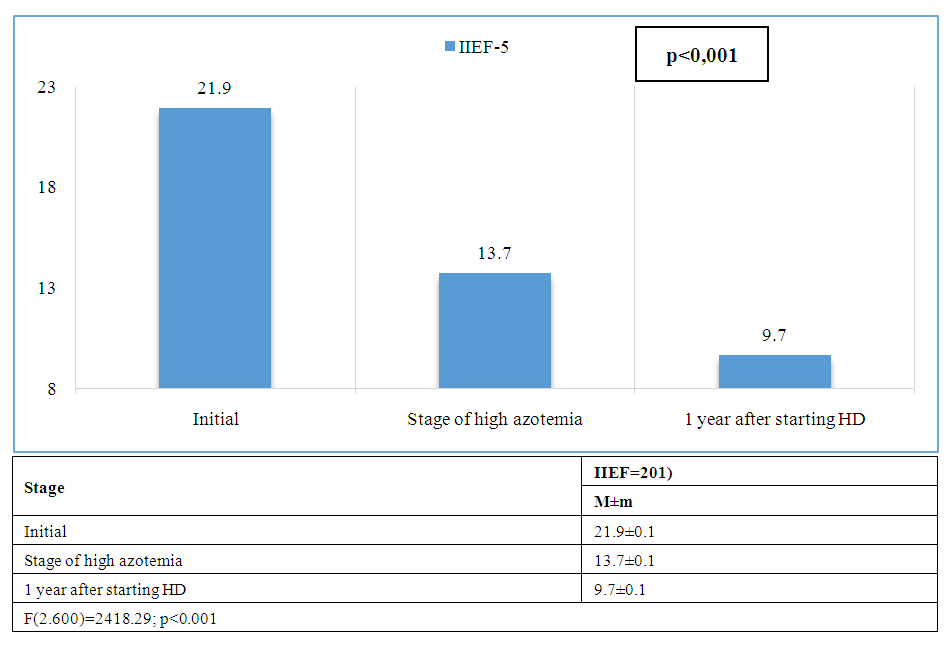 | Figure 1. Dynamics of average values of the International Index of Erectile Function (IIEF-5) |
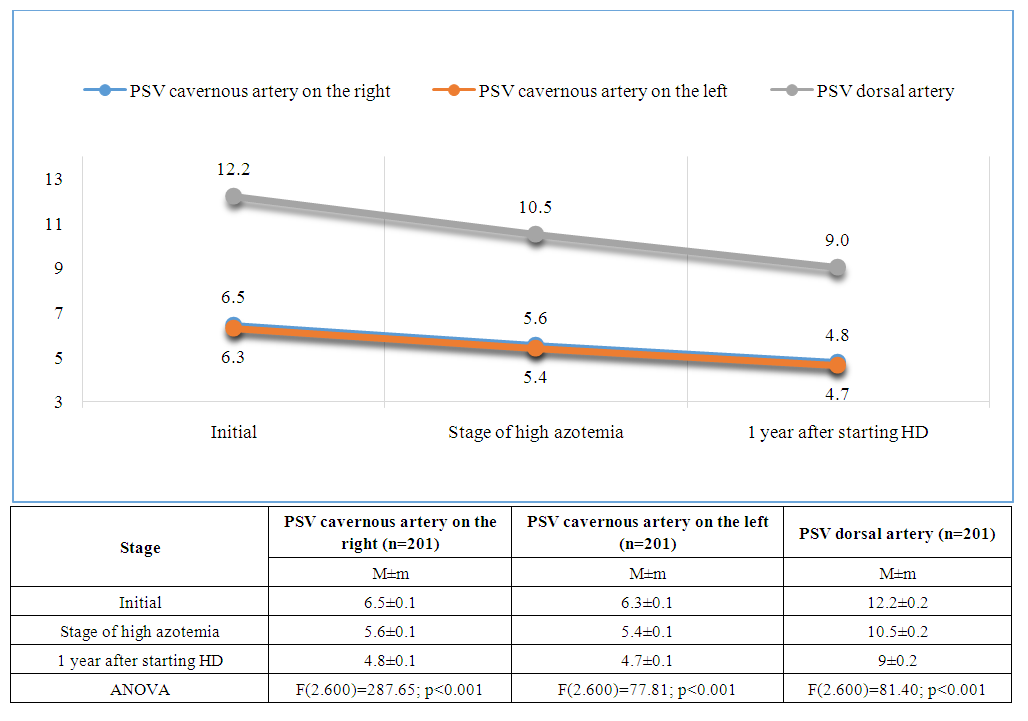 | Figure 2. Dynamics of peak systolic velocity in the cavernous arteries (right and left) and the dorsal artery of the penis |
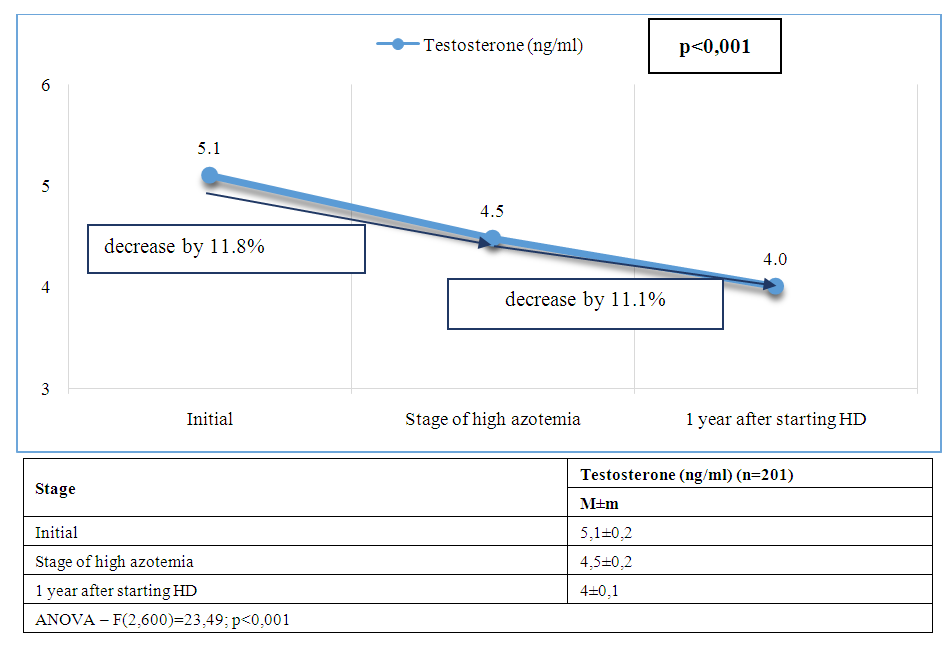 | Figure 3. Dynamics of average testosterone levels at stages of management of patients with CKD and ED |
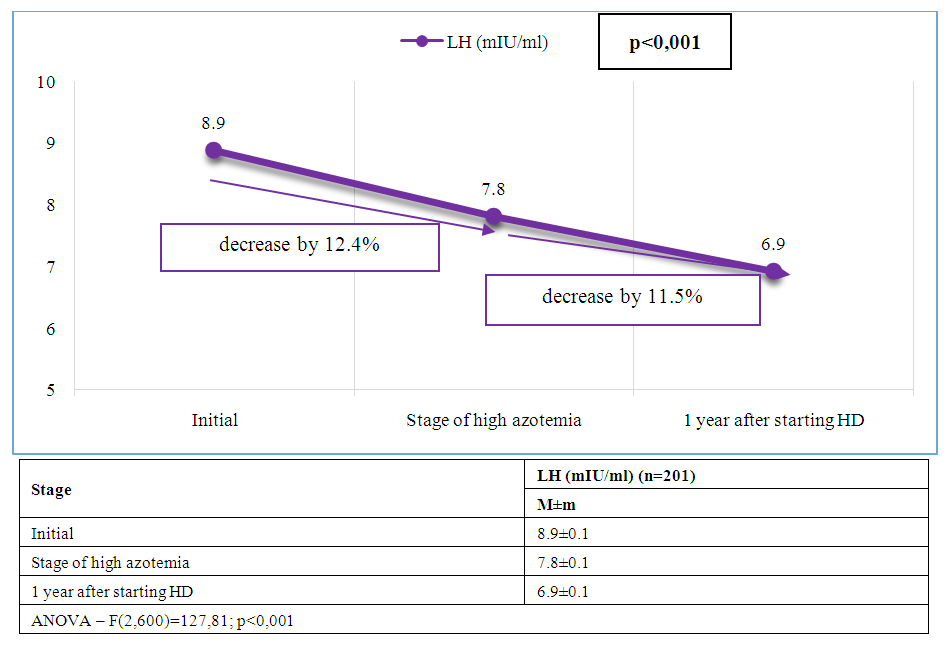 | Figure 4. Dynamics of average LH levels at stages of management of patients with CKD and ED |
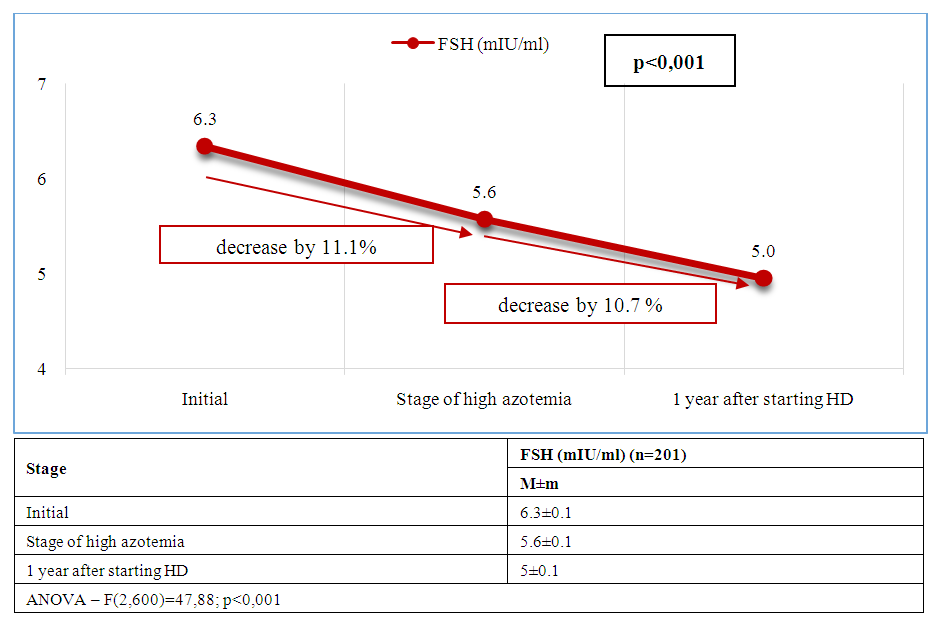 | Figure 5. Dynamics of average FSH levels at stages of management of patients with CKD and ED |
4. Discussion
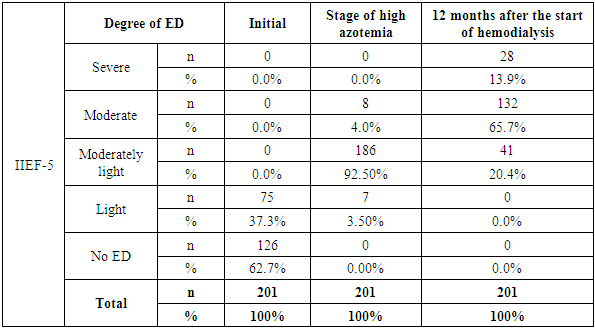
- Reproductive disorders in patients with chronic kidney disease represent one of the most complex and understudied areas of nephrology and transplantology. Current research indicates a high prevalence of sexual dysfunction, hypogonadism, infertility and decreased libido in patients suffering from terminal CKD. The main pathogenetic mechanisms of sexual dysfunction at CKD are chronic intoxication with uremic toxins, anemia, hyperprolactinemia, electrolyte disorders, as well as secondary hyperparathyroidism and disorders of hypothalamic-pituitary-gonadal axis function. Impaired metabolism of sex hormones, decreased testosterone in men and estrogens in women are closely related to the level of glomerular filtration and become especially pronounced with the transition to programmed hemodialysis.Most frequently it associated with the use of glucocorticosteroids, calcineurin inhibitors, and antimetabolites, which can adversely affect spermatogenesis, libido and erectile function. In our presented study it was revealed that the severity of reproductive disorders depends not only on the stage of CKD and duration of replacement therapy, but also on individual factors such as age, presence of concomitant endocrine and cardiovascular diseases, etc. Thus, erectile dysfunction (IIEF-5) is closely associated with impaired blood flow in the cavernous arteries (PSV) and decreased testosterone. Hypogonadism (low levels of testosterone, LH, FSH) leads to deterioration of spermogram parameters, vascular disorders (decreased PSV) worsen erectile function.Based on the conducted correlation analysis, multivariate analysis (linear regression) and statistical processing of data, it can be concluded that ED in CKD has a mixed genesis, including vascular (arteriogenic or impaired veno-occlusive mechanism), hormonal (endocrine) and mixed components.Particular attention should be paid to the need for a multidisciplinary approach in the evaluation and correction of reproductive disorders in this type of patients. Comprehensive evaluation of the hormonal profile, ultrasound and laboratory examination, as well as timely consultation with a urologist-andrologist and endocrinologist can increase the chances of restoring reproductive function and improving the life quality of patients.
5. Conclusions
- Reproductive disorders in patients with CKD are a common and significant problem affecting not only the quality of life but also the long-term outcome of the disease. Patients with CKD undergoing hemodialysis have been found to have significant endocrine and vascular disorders leading to reproductive disorders, including hypogonadism and ED. Analysis of erectile function in patients with CKD and dialysis therapy showed a progressive deterioration of sexual function with increasing azotemia and duration of HD.
Conflict of Interests’ Statement
- The authors declare no conflict of interest. This study does not include the involvement of any budgetary, grant or other funds. The article is published for the first time and is part of a scientific work.
ACKNOWLEDGEMENTS
- The authors express their gratitude to the management of the multidisciplinary clinic of Republican Specialized Scientific and Practical Medical Center of Urology for the material provided for our study.
Ethical Approval and Consent to Participate
- The Research Ethics Board of our institution does not require review or approval of case reports. Our research was carried out in accordance with the World Medical Association Code of Ethics (Declaration of Helsinki).
Source of Funding
- Each of the authors has reviewed and approved this manuscript. None of the authors has a conflict of interest, financial or otherwise. This manuscript is original, no part of it has been previously published and is not being considered for publication elsewhere. The corresponding author agrees to accept full responsibility for authorship at the submission and review stages of the manuscript.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML