M. M. Aliev1, U. Kh. Tilavov1, F. F. Boyakhmedov1, Kh. Kh. Sultanov2, A. M. Auyesov2
1Department of Pediatric Elective Surgery, Tashkent Pediatric Medical Institute, Tashkent, Uzbekistan
2National Medical Center for Children, Tashkent, Uzbekistan
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Background: Bronchopulmonary malformations (BPMs) are rare but clinically significant congenital anomalies of the lungs and bronchi that may present in infancy or later childhood. The diagnostic and therapeutic management of BPMs is often challenging due to the heterogeneity of presentations and the lack of standardized classification systems. Objective: To evaluate modern diagnostic approaches and the effectiveness of minimally invasive, organ-preserving surgical interventions in children with BPMs. Materials and Methods: A retrospective study was conducted on 146 pediatric patients (aged 2 months to 18 years) treated for BPMs between 2004 and 2024 at TashPMI Clinic and the National Children's Medical Center. Diagnostic tools included chest radiography, MSCT angiography, bronchoscopy, bronchography, echocardiography, and selective angiopulmonography. BPMs were classified using the S. Michael system (2017). Results: Pulmonary hypoplasia was the most common anomaly (52.7%), predominantly affecting the right lung. Surgical interventions varied by malformation type and included thoracotomy (lobectomy, bilobectomy, pneumonectomy), segmental resections, bronchial occlusions, and thoracoscopic procedures. Postoperative complications occurred in 17% of patients undergoing resection. Minimally invasive procedures demonstrated fewer complications and better functional outcomes, especially in cases identified and treated before the age of 6. Conclusion: Timely diagnosis and individualized surgical strategy, including the use of MSCT angiography and bronchography, are crucial in managing BPMs. Thoracoscopic resection and selective bronchial occlusion are effective in organ preservation. Early surgical intervention significantly improves long-term outcomes in children with BPMs.
Keywords:
Bronchopulmonary malformations
Cite this paper: M. M. Aliev, U. Kh. Tilavov, F. F. Boyakhmedov, Kh. Kh. Sultanov, A. M. Auyesov, Diagnostics and Treatment of Bronchopulmonary Malformations in Children, American Journal of Medicine and Medical Sciences, Vol. 15 No. 7, 2025, pp. 2328-2333. doi: 10.5923/j.ajmms.20251507.51.
1. Introduction
Broncho-pulmonary malformations (BPM) are anatomical and morphological deviations from the norm that arise due to disturbances in the embryonic development of the fetus or at later stages of organ formation, including the postnatal period [6,8,10,11,13,15].For pediatricians and pediatric surgeons, developmental pathology of the broncho-pulmonary system remains a relevant issue. According to various authors, BPM accounts for 2% to 64% of the causes of chronic inflammatory diseases of the respiratory system [2,4,12,16].Such a wide variation in statistical data is explained by the lack of a unified classification, difficulties in distinguishing between congenital and acquired pathology, and the complexity of choosing treatment tactics and surgical approaches, especially in the presence of pronounced inflammatory processes [1,3,9,14].The aim of the study is to apply modern diagnostic methods and analyze the effectiveness of organ-preserving, minimally invasive surgical interventions in children with broncho-pulmonary malformations.
2. Materials and Methods
This scientific study is based on the examination and treatment results of 146 children aged from 2 months to 18 years with bronchopulmonary malformations (BPM), who were hospitalized in the clinics of TashPMI and BMTM from 2004 to 2024. Alongside general clinical examination methods, instrumental diagnostic techniques were also used for patient evaluation. These included standard chest X-rays in two projections, MSCT angiography, spirometry, bronchoscopy, bronchography, echocardiography (EchoCG), Doppler ultrasound of the pulmonary arteries, and angiopulmonography.In this study, we used the latest classification developed by S. Michael and co-authors (USA, Canada, 2017) and included the most frequently encountered BPM types in our experience. These are congenital developmental defects of the bronchi and lung parenchyma related to surgical pathology: congenital malformations of the pulmonary airways (CPAM), congenital lobar emphysema (CLE), pulmonary hypoplasia: lung aplasia (LA), simple (SH) and cystic (CH) forms, hypoplastic (postnatal) bronchiectasis (HB) (see Figure 1).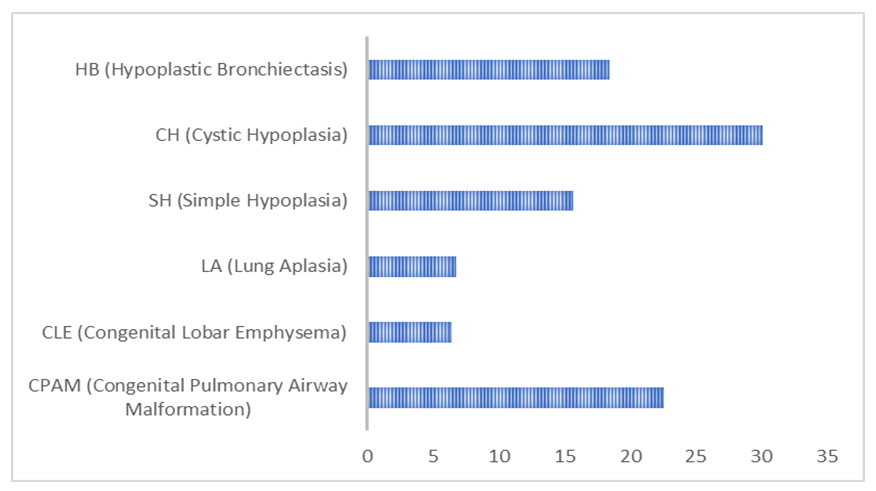 | Figure 1. Distribution of patients by nosological forms (n=146) |
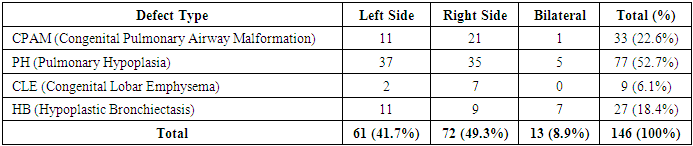
According to Table 1, the majority of patients were diagnosed with pulmonary hypoplasia (PH) and right-sided lesions. These accounted for 52.7% and 49.3% of the total patient number, respectively. Among the sick children, boys were the majority—83 patients (56.8%), while girls numbered 63 (43.2%).Table 1. Distribution by nosological forms and affected side
 |
| |
|
BPM was most commonly diagnosed in children aged 7–11 years, with 36 patients (24.6%). Unilateral single-lobe lesions were found in 93 patients (63.6%), bilateral two-lobe lesions in 16 patients (10.9%), isolated segmental lesions in 23 patients (15.7%), and bilateral lesions in 14 patients (9.6%).
3. Results and Discussion
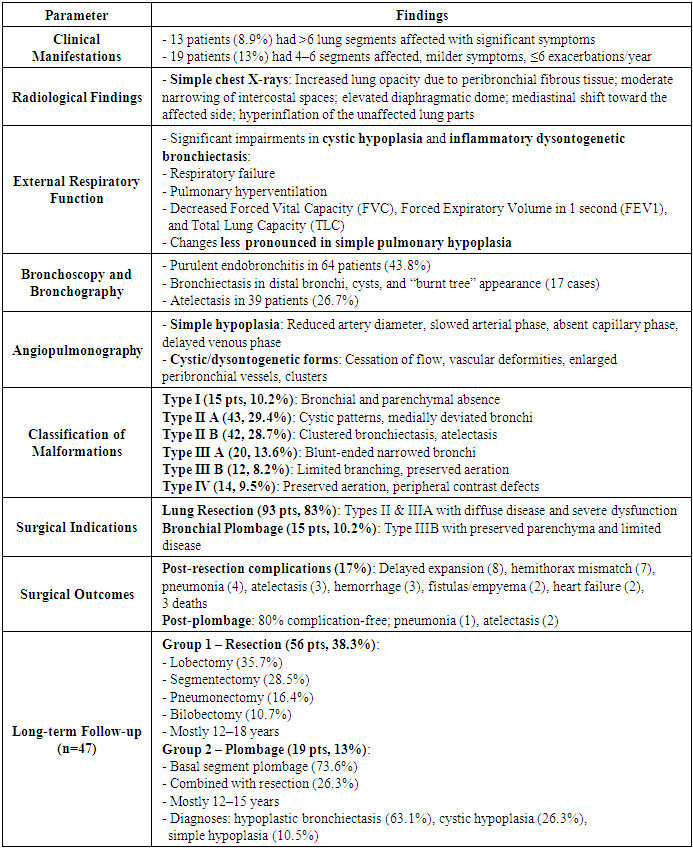
The clinical presentation of the disease was often characterized by recurrent bronchopneumonia symptoms. Significant clinical manifestations were observed in 13 patients (8.9%) who had unilateral or bilateral involvement of more than 6 lung segments.Among 19 patients (13%) with 4-6 affected segments, disease recurrence was less frequent (no more than 4-6 times per year). Their overall condition was relatively satisfactory, with fewer signs of purulent intoxication, and respiratory failure was observed only during noticeable physical exertion. Cough was mild, and sputum production was moderate.On simple chest X-rays of the affected side, there was increased lung opacity due to peribronchial fibrous tissue, moderate narrowing of intercostal spaces, elevated diaphragmatic dome, varying degrees of mediastinal shift toward the affected side, and hyperinflation of the unaffected lung parts. As shown in Table 2, significant changes in external respiratory function were typical during the cystic form of the disease and during inflammatory exacerbations of dysontogenetic bronchiectasis in children: respiratory failure, pulmonary hyperventilation, decreased forced vital capacity (FVC), forced expiratory volume (FEV1), and total lung capacity (TLC). These changes were less pronounced in simple pulmonary hypoplasia.Table 2. Results and Discussion – Clinical, Radiological, and Surgical Features of Bronchopulmonary Malformations (BPM) in Children
 |
| |
|
Diagnostic bronchoscopy revealed signs of purulent endobronchitis in 64 patients (43.8%). Bronchography identified uniformly dilated cyst-like structures, and bronchiectasis was detected in the distal small bronchi. Segmental and subsegmental bronchi often showed incomplete contrast filling; fourth and fifth order bronchi ended in irregular-shaped cysts. Architectural changes in bronchi included reduced branching angles and bronchi clustered in “bundles” or “clusters,” with all bronchial branches in these clusters being uniformly dilated and deformed. Atelectasis of lung parenchyma was observed in 39 patients (26.7%). In cases with unaffected lung parenchyma, segmental bronchi of a single lobe were not dilated but chronic bronchitis signs were found in adjacent segmental bronchi (18 cases). The simple form of the disease was characterized by narrowing, irregular contour, and significantly reduced size of affected lobes and segmental bronchi. Distal bronchi were bluntly closed, and bronchograms showed a “burnt tree” appearance (17 cases).In simple hypoplasia, selective angiopulmonography showed that the pulmonary artery on the affected side was contrasted, with a reduced diameter and smaller arterial branches; arterial phase was slowed, capillary phase absent, and venous phase significantly delayed. In cystic hypoplasia and dysontogenetic bronchiectasis, vascular changes included complete cessation of blood flow at times, sharp bends in bronchial arteries indicating increased peribronchial blood flow; often, large and small vascular conglomerates formed, merged, and connected in different parts of the hypoplastic lung; marked vascular deformations were noted, including narrowed internal diameters, changes in direction, and weakened contrast enhancement.Based on the clinical, radiological, and instrumental data from the patients diagnosed with bronchopulmonary malformations (BPM), we conditionally classified malformations into four types to avoid confusion in classification and treatment approaches:Type I (15 patients - 10.2%)Radiological: Hemithorax shadowing, elevated diaphragmatic dome, mediastinal shift toward affected side, narrowed intercostal spaces, emphysematous expansion of the healthy lung.Bronchographic: Absence of central bronchial tree filling.Tomographic: Absence of central and peripheral bronchi, atelectasis or absence of lung parenchyma, elevated diaphragm, mediastinal shift, emphysematous expansion of the healthy lung.Type II “A” (43 patients - 29.4%)Radiological: Reduced lung aeration with cystic light areas lacking clear capsules.Bronchographic and tomographic: Elongation and narrowing of subsegmental bronchi with ring-shaped light areas on shadowing background; no cyst filling during bronchography; bronchi of the third to fourth order were deformed, often deviated medially.Type II “B” (42 patients - 28.7%)Architectural changes in bronchi of the third to fifth order: reduced branching angles, bronchi closely clustered in “bunches.” All bronchial branches in the “bunch” were enlarged and uniformly deformed. Distal small bronchi often transformed into bronchiectasis or small cysts. The affected lung part was atelectatic.Type III “A” (20 patients - 13.6%)Bronchographic and tomographic: Marked narrowing of lobar bronchus with shadowing of lung area; elongated, narrowed, and blunt-ended segmental or subsegmental bronchi.Type III “B” (12 patients - 8.2%)Bronchial architecture changes: narrowed segmental bronchi with limited branching; straight direction reaching the pleura, with small branches dividing off. Some subsegmental bronchi were dilated and blunt-ended but lung parenchyma in malformation area was aerated.Type IV (14 patients - 9.5%)Preserved aeration in affected lung parts; peripheral parts of some bronchial branches were not contrasted.Indications for lung resection included clear clinical presentation of disease, significant reduction of lung transparency on radiograms, diffuse purulent endobronchitis, bronchograms showing open and near bronchi, and angiopulmonograms demonstrating reduced branching angles of arterial branches, their patency, and marked decrease in capillary phase imaging in 93 patients (83%) – radiological types II and IIIA.Indications for bronchial plombage were mild clinical manifestations (few purulent intoxications and recurrent bronchopneumonia), absence of focal or infiltrative shadows on radiograms, predominance of limited or partially diffuse catarrhal and purulent-catarrhal endobronchitis on bronchoscopy, alveolar airway contrast in bronchograms, and minimal vascular changes in angiopulmonograms (preserved capillary phase – radiological type III-B). Evaluation of lung parenchyma intraoperatively during hyperventilation and palpation showed preserved aeration and elasticity in 19 patients (13.0%). Bronchial plombage of affected segments was performed intraoperatively in 15 patients (10.2%). Lung resection combined with bronchial plombage was done in 26.3% of cases. Plombage most commonly targeted bronchi of the lower lobe on the left side (21%), less often on the right (5.2%).Postoperative complications were recorded in 17% of children after lung resection surgery. The most common complications were delayed lung collapse and expansion (8 cases). Seven cases had mismatch between hemithorax and remaining lung tissue volume after bilobectomy. Atelectasis and pneumonia of operated lung occurred in 3 and 4 patients respectively, mainly due to purulent bronchitis. Other complications included intrapleural hemorrhage (3 patients), bronchial fistulas and pleural empyema (2 patients), and pulmonary heart failure (2 patients).Three patients died in the early postoperative period.Following bronchial plombage surgery, 80% of patients had no postoperative complications, and their general condition was satisfactory. Postoperative pneumonia was observed in 1 patient, and partial atelectasis of plombaged lobe in 2 patients.Long-term outcomes (6 months to 14 years) were studied in 47 patients. Based on surgical treatment methods, all patients were divided into three groups:Group 1 (56 patients, 38.3%): underwent lobar, bilobar, pneumonectomy, or segmental lung resection via thoracotomy. Lobectomy was the most frequent surgery in this group (20 patients, 35.7%), followed by segmentectomy (16 patients, 28.5%), pneumonectomy (9 patients, 16.4%), and bilobectomy (6 patients, 10.7%). The majority of patients in this group were aged 12-18 years (28.5%), likely due to late diagnosis and low referral rates to specialized centers.Group 2 (19 patients, 13%): underwent bronchial plombage of affected lung parts. Basal segment plombage was performed in 73.6% of cases; combined plombage and resection of the IV–V segments was done in 26.3%. The largest age group was 12–15 years (52.6%). Bronchial plombage in simple hypoplasia was done in 2 patients (10.5%) and cystic hypoplasia in 5 patients (26.3%). Hypoplastic bronchiectasis was present in 12 patients (63.1%).Group 3 (37 patients, 25.3%): underwent total or partial lung resection via thoracoscopic surgery (see Fig. 2).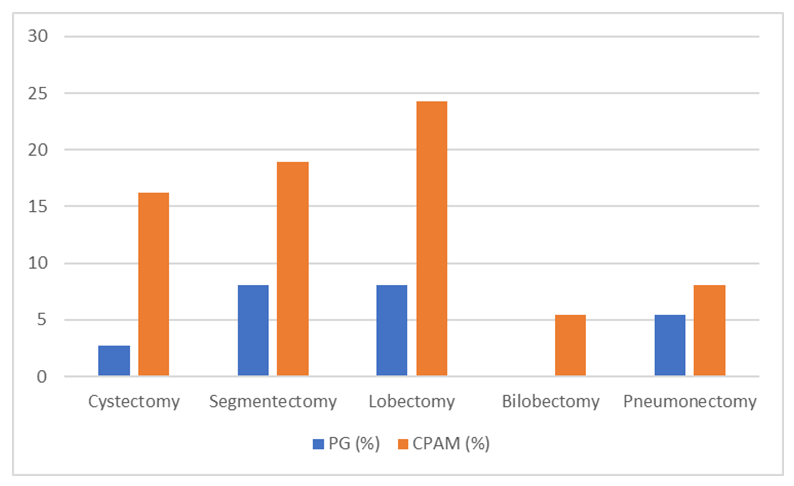 | Figure 2. Types of VATS Lung Resection According to Malformation Form |
Among the children who underwent surgery in this group, the largest share was lobectomy — performed in 13 patients (35.7%). Segmentectomy, cystectomy, and pneumonectomy were performed in 9 (24.3%), 8 (21.6%), and 5 (13.5%) patients, respectively. The majority of patients in the third group were aged 12–15 years (31.8%). The predominance of patients aged 7–15 years is explained by late diagnosis and less frequent referral to specialized medical institutions.Thus, bronchological and MSCT angiographic studies are conditionally performed, which allow assessment of the degree of pneumatisation of the affected segments and the efficiency of blood flow. In determining the surgical intervention method for congenital lung malformations (CLM), intraoperative visual and palpation evaluation of lung tissue pneumatisation and blood flow is crucial. Due to the proximal branching type of bronchi (IV-V) and the impossibility of re-aeration of atelectatic areas as well as a significant reduction in blood flow in lung tissue, resection of the affected lung tissue is inevitable. In other cases, i.e., if re-aeration is possible and the capillary phase of blood flow is preserved, sealing (plombage) of the bronchi of the affected lobe or segments is recommended.Long-term postoperative analysis showed the following results: good — 17 patients (36.1%), satisfactory — 21 patients (44.6%), unsatisfactory — 6 patients (12.7%). Unsatisfactory long-term results were associated with the progression of inflammatory processes in extensive and bilateral malformation areas.
4. Conclusions
MSCT angiography is sufficient for diagnosing CPAM, pulmonary hypoplasia, and congenital cystic adenomatoid malformation (CCAM), but selective bronchography is necessary for bronchial atresia, bronchiectasis, and hypoplastic bronchiectasis to select treatment tactics. These are chosen depending on the conditional forms of CLM.Surgical operations are performed in the second and third forms of CLM.Thoracoscopic resection (in CLM forms 2A, 2B, and 3A) and sealing (plombage) of the affected lung part in CLM form 3B are the most radical and effective treatments.Achieving good surgical outcomes strongly depends on the timing of surgical intervention (preferably before 6 years of age), which is largely determined by early diagnosis.Subsequent lung resections performed due to extensive purulent processes increase the volume of lung resection and are accompanied by a higher risk of recurrent purulent inflammation.
References
| [1] | Ovsjannikov DJu, Frolov PA, Semenov PA. Vrozhdennaya mal’formaciya dykhatel’nykh putej. Pediatriya. 2018; 97(1): 152–161. |
| [2] | Razumovskij AYu, et al. Mini-invazivnaya khirurgiya v lechenii detej s kistozno-adenomatoznoj mal’formaciej legkikh. Detskaya khirurgiya. 2013; (2): 4–8. |
| [3] | Tashchilkina YuV, et al. Vrozhdennaya kistozno-adenomatoznaya mal’formaciya legkogo III tipa (klinicheskij sluchaj). Vizualizaciya v medicine. 2023; 5(2): 32–37. |
| [4] | Shestak EV. Kistozno-adenomatoznaya mal’formaciya legkogo II tipa u novorozhdennogo, problemy rannej diagnostiki. Uralskij medicinskij zhurnal. 2022; 21(1): 77–84. doi:10.52420/2071-5943-2022-21-1-77-84. |
| [5] | Bakhuizen JJ, Postema AM, van Rijn RR, Schuppen J, et al. No pathogenic DICER1 gene variants in a cohort study of 28 children with congenital pulmonary airway malformation. J Pediatr Surg. 2024; 59(3): 459–463. |
| [6] | Ottomeyer M, et al. Early resection of a rare congenital pulmonary airway malformation causing severe progressive respiratory distress in a preterm neonate: a case report and review of the literature. BMC Pediatr. 2023; 23: 238. doi:10.1186/s12887-023-03991-z. |
| [7] | Goussard P, et al. Bronchoscopy findings in children with congenital lung and lower airway abnormalities. Pulm Rev. 2023. doi:10.1016/j.prrv.2023.10.01. |
| [8] | Palla J, Sockrider MM. Congenital lung malformations. Pediatr Ann. 2019; 48(4): e169–e174. doi:10.3928/19382359-20190326-02. |
| [9] | Patrick T, et al. Pediatric congenital lung malformations imaging guidelines and recommendations. Radiol Clin North Am. 2022; 60: 41–54. doi:10.1016/j.rcl.2021.08.002. |
| [10] | Rothenberg SS. Thoracoscopic lobectomy in infants and children. J Laparoendosc Adv Surg Tech A. 2021; 31(10): 1157–1161. |
| [11] | Seear M, Townsend J, Hoepker A, et al. A review of congenital lung malformations with a simplified classification system for clinical and research use. Pediatr Surg Int. 2017; 33(6): 657–664. |
| [12] | Seohee J, et al. Thoracoscopic segmentectomy in children with congenital lung malformation. Sci Rep. 2023; 13: 9640. doi:10.1038/s41598-023-36700-5. |
| [13] | Stocker LJ, et al. The increasing incidence of foetal echogenic congenital lung malformations: an observational study. Prenat Diagn. 2015; 35: 148–153. |
| [14] | Yamataka A, Koga H, Ochi T, et al. Pulmonary lobectomy techniques in infants and children. Pediatr Surg Int. 2017; 33(5): 483–495. |
| [15] | Zeng J, Liang J, Li L, et al. Surgical treatment for asymptomatic congenital pulmonary airway malformations in children: waiting or not? Eur J Pediatr Surg. 2021; 31(6): 509–517. |
| [16] | Zheng J, et al. Thoracoscopic versus open resection for symptomatic congenital pulmonary airway malformations in neonates: a decade-long retrospective study. BMC Pulm Med. 2021; 21: 82. doi:10.1186/s12890-021-01445-2. |




 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML
