-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(7): 2258-2260
doi:10.5923/j.ajmms.20251507.36
Received: Jun. 12, 2025; Accepted: Jul. 6, 2025; Published: Jul. 11, 2025

Features of Cardiomyopathy Progression in Patients with Type 2 Diabetes Living in Hot Climates
F. A. Ikramova
Bukhara State Medical University, Bukhara, Uzbekistan
Correspondence to: F. A. Ikramova, Bukhara State Medical University, Bukhara, Uzbekistan.
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The purpose of the research: This study aims to assess the clinical and functional characteristics of diabetic cardiomyopathy (DCM) in patients with type 2 diabetes mellitus (T2DM) who reside in regions with hot climates and to identify strategies for optimizing metabolic therapy under these conditions. A cohort of 180 patients with T2DM was observed in a regional cardiology center. Clinical, echocardiographic, electrocardiographic, and laboratory evaluations were performed. Patients were divided into two groups depending on the presence or absence of DCM. Statistical analysis was conducted using SPSS v25.0. Patients with DCM showed significantly more complaints of dyspnea (74%), fatigue (68%), and edema (61%). Diastolic dysfunction (E/A < 1.0) was identified in 71% of DCM patients, while GLS < -17% was observed in 64%. HRV was reduced (SDNN 91 ± 11 ms), and arrhythmias occurred in 38% of cases. HbA1c and NT-proBNP levels were elevated. Electrolyte imbalances were observed in 62% of DCM patients. The results support the necessity of climate-adapted therapy for T2DM patients, including adjusted hydration strategies, greater use of cardioprotective antidiabetics, and enhanced patient education on heat stress prevention.
Keywords: Type 2 diabetes mellitus, Diabetic cardiomyopathy, Hot climate, Echocardiography, Arrhythmia, Metabolic therapy, Cardiac function
Cite this paper: F. A. Ikramova, Features of Cardiomyopathy Progression in Patients with Type 2 Diabetes Living in Hot Climates, American Journal of Medicine and Medical Sciences, Vol. 15 No. 7, 2025, pp. 2258-2260. doi: 10.5923/j.ajmms.20251507.36.
1. Introduction
- Type 2 diabetes mellitus (T2DM) is one of the most widespread chronic diseases worldwide, and its socio-economic burden continues to grow. Current epidemiological data indicate a significant increase in T2DM incidence in regions with hot climates, particularly in the Middle East, Central Asia, Southern Europe, and North Africa. These climatic conditions exacerbate the course and complications of T2DM [1,2,3].Among the complications, diabetic cardiomyopathy (DCM) plays a significant role. This condition is characterized by structural and functional changes in the myocardium, developing in the absence of coronary artery disease, hypertension, or valvular defects. DCM significantly increases the risk of heart failure, sudden cardiac death, and reduces life expectancy [5,7,9].Hot climates impose additional stress on the cardiovascular system of patients with T2DM. High temperatures contribute to dehydration, electrolyte imbalance, increased blood viscosity, and activation of the sympathoadrenal system, all of which can aggravate cardiomyopathy and increase the risk of decompensation [4,11,12].Despite numerous studies on DCM pathogenesis and treatment, the impact of climatic factors, particularly extreme heat, remains underexplored. It is especially important to consider how to adapt therapeutic approaches under high ambient temperatures [6,10].ObjectiveTo identify the clinical and functional features of diabetic cardiomyopathy in patients with T2DM living in hot climates, and to determine the directions for therapy adaptation under such conditions.
2. Materials and Methods
- The study was conducted at a regional cardiology center located in a southern climatic zone. A total of 180 patients with T2DM living in hot climates for at least 5 years were included. All patients provided informed consent.Inclusion criteria: age 45–70 years, T2DM duration over 5 years, no significant hypertension, coronary artery disease, or structural heart defects.The patients were divided into two groups: Group A (n=90) – patients with signs of DCM confirmed via echocardiography and lab tests; Group B (n=90) – patients without DCM signs.Study methods included:- Clinical and anamnestic evaluation (complaints, NYHA class, BMI);- Echocardiography (EF, diastolic function, GLS);- 24-hour ECG monitoring (HRV, arrhythmias);- Lab tests (HbA1c, NT-proBNP, electrolytes, inflammation markers);- Treatment scheme assessment and compliance.
3. Results
- The study revealed the following patterns:
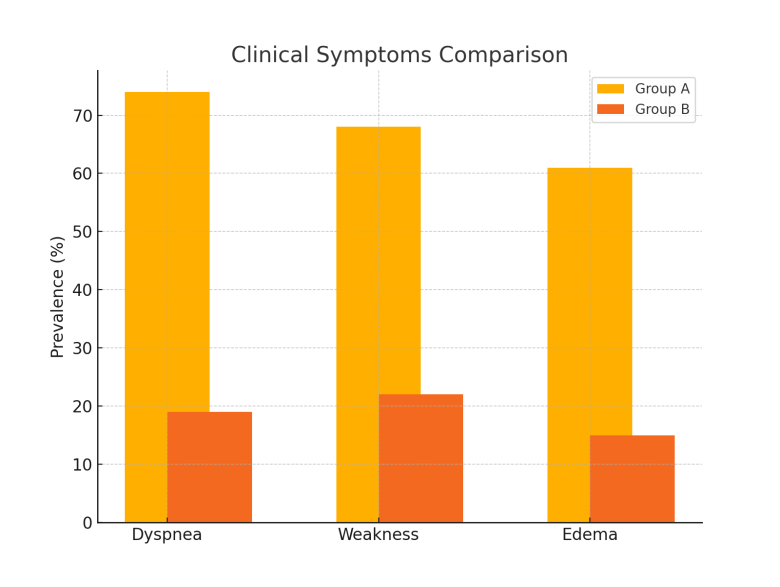 | Figure 1. Comparison of clinical symptoms between groups |
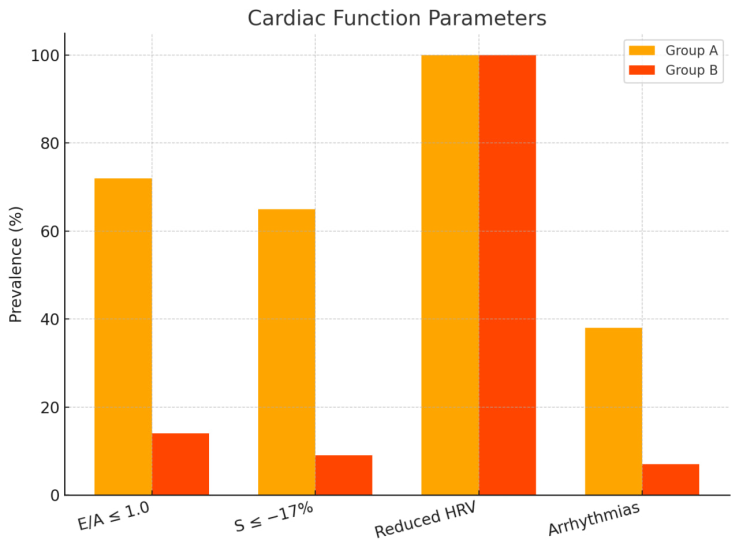 | Figure 2. Heart function measures in patients with DCM |
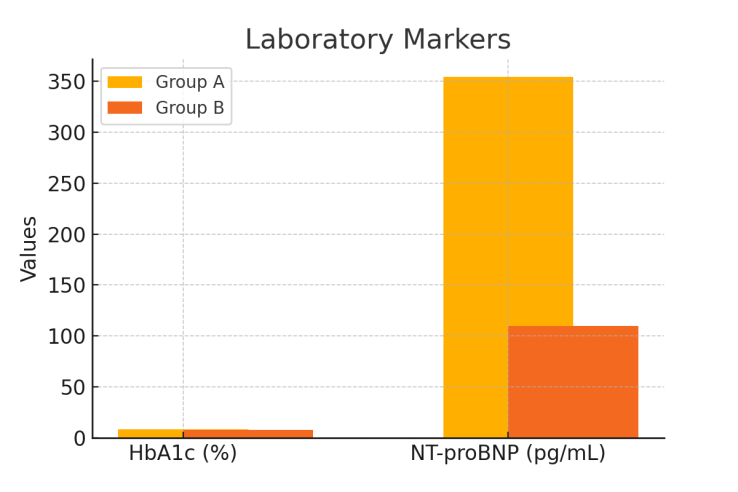 | Figure 3. Laboratory markers in Group A and Group B |
4. Conclusions
- Patients with T2DM living in hot climates experience a more severe course of diabetic cardiomyopathy. Major aggravating factors include dehydration, reduced circulating blood volume, electrolyte imbalance, elevated body temperature, and sympathoadrenal activation.Our results support therapy adaptation: increased use of SGLT2 inhibitors, dose adjustments of diuretics, regular monitoring of electrolytes and hydration. Patient education on hydration and self-monitoring is especially important during hot seasons.
5. Future Directions
- Future studies should focus on multicenter validation, including gender and age analysis, comorbidities, and evaluation of personalized prevention programs for complications in this patient population.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML