-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(7): 2244-2246
doi:10.5923/j.ajmms.20251507.32
Received: Jun. 12, 2025; Accepted: Jul. 8, 2025; Published: Jul. 11, 2025

Results of Immunochemohormonal Therapy in Breast Cancer Patients with Liver Metastases
Rakhimov Nodir Makhammatkulovich1, Eshmurodov Uktam Makhmudovich2, Shakhanova Shakhnоza Shavkatovna3
1DSc., Professor, Department of Oncology, Samarkand State Medical University, Samarkand, Uzbekistan
2Samarkand State Medical University, Samarkand, Uzbekistan
3PhD., Associate Professor, Department of Oncology, Samarkand State Medical University, Samarkand, Uzbekistan
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

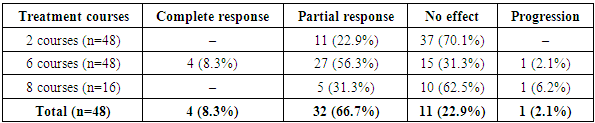
This article presents the results of comprehensive immunochemohormonal therapy in 48 patients with breast cancer (BC) with liver metastases and luminal A subtype. Treatment included polychemotherapy according to the PIAF regimen (cisplatin, fluorouracil, doxorubicin) combined with interferon-α and hormonal therapy using palbociclib with aromatase inhibitors. Treatment effectiveness was evaluated after the 2nd, 6th, and 8th courses. A complete response was achieved in 8.3% of patients, a partial response in 66.7%, no response in 22.9%, and disease progression occurred in 2.1%. In 25% of cases, therapy was ineffective due to the development of multiple drug resistance. The median survival was 16.5 months; one-year survival rate was 71.4%, two-year — 25.7%, three-year — 14.3%, and four-year — 5.7%. Only two patients remained alive without signs of disease progression two years after treatment. These findings confirm the limited but clinically meaningful effectiveness of a combined approach in the management of aggressive forms of metastatic breast cancer.
Keywords: Breast cancer, Liver metastases, Immunotherapy, Hormonal therapy, Polychemotherapy, PIAF, Palbociclib, Luminal A subtype, Survival, Multidrug resistance
Cite this paper: Rakhimov Nodir Makhammatkulovich, Eshmurodov Uktam Makhmudovich, Shakhanova Shakhnоza Shavkatovna, Results of Immunochemohormonal Therapy in Breast Cancer Patients with Liver Metastases, American Journal of Medicine and Medical Sciences, Vol. 15 No. 7, 2025, pp. 2244-2246. doi: 10.5923/j.ajmms.20251507.32.
1. Introduction
- Breast cancer remains the leading cause of cancer-related mortality worldwide. Approximately 50% of all women diagnosed with breast cancer develop distant metastases to organs such as the liver, lungs, bones, and brain [3,7]. Liver metastases occur in about 50% of all patients with metastatic breast cancer (MBC), and in 5–12% of cases, the liver is the primary site of metastatic involvement at the time of diagnosis [1,4]. Hepatic metastases can lead to significant impairment of liver function, which negatively impacts the prognosis of patients with breast cancer. In the absence of treatment, liver metastases are associated with poor survival outcomes, with median survival ranging from 4 to 8 months [2,5]. Moreover, despite advances in systemic therapy, the average life expectancy of patients with liver metastases from the time of diagnosis remains between 18 and 24 months, while 5-year and 10-year survival rates are still low—27% and 13%, respectively [8]. Therefore, a personalized approach to the treatment of liver metastases, based on the individual prognosis of the disease, underscores the relevance of this research [5,9].
2. Materials and Methods
- This treatment approach was applied to 48 patients with breast cancer (BC) who had liver metastases and luminal A subtype tumors. The immunotherapy component was aimed at restoring and supporting the patient’s immune defenses. This therapy helps to reactivate the immune system and nonspecific antitumor mechanisms, which contribute to the long-term effectiveness of the treatment and the potential elimination of cancer cells from the body.Immunotherapy was administered in combination with polychemotherapy following the PIAF regimen: Cisplatin 20 mg/m² on days 1–4, Fluorouracil 400 mg/m² on days 1–4, Doxorubicin 40 mg/m² on day 1, and Interferon-α 5 million IU/m² subcutaneously on days 1–4. The interval between treatment courses was three weeks.Hormonal therapy included the use of Palbociclib in combination with aromatase inhibitors.
3. Discussion
- Among the side effects observed during treatment, the most common were flu-like symptoms lasting 24–36 hours, nausea, vomiting, loss of appetite, diarrhea, dizziness, blood pressure instability, tachycardia, musculoskeletal pain, hot flashes, depressed mood, and insomnia.Clinical and biochemical analyses revealed reductions in erythrocyte and platelet counts, decreased prothrombin index (PTI), elevated fibrinogen levels, and increases in liver enzymes (ALT, AST), lactate dehydrogenase (LDH), and alkaline phosphatase (ALP). Mild increases in indirect bilirubin levels were noted in some patients.Treatment efficacy in this cohort was assessed after the 2nd, 6th, and 8th treatment courses. In patients showing a positive response and satisfactory general condition, therapy was extended to 8 cycles. In cases of poor general condition, therapy was discontinued after 6 cycles, and patients were managed on an outpatient basis.As shown in Table 1, 16 patients underwent 8 courses of therapy, while treatment was discontinued after 6 courses in 32 patients. In 12 patients (25%), treatment was ineffective even after 8 courses, primarily due to the development of multiple drug resistance.
|
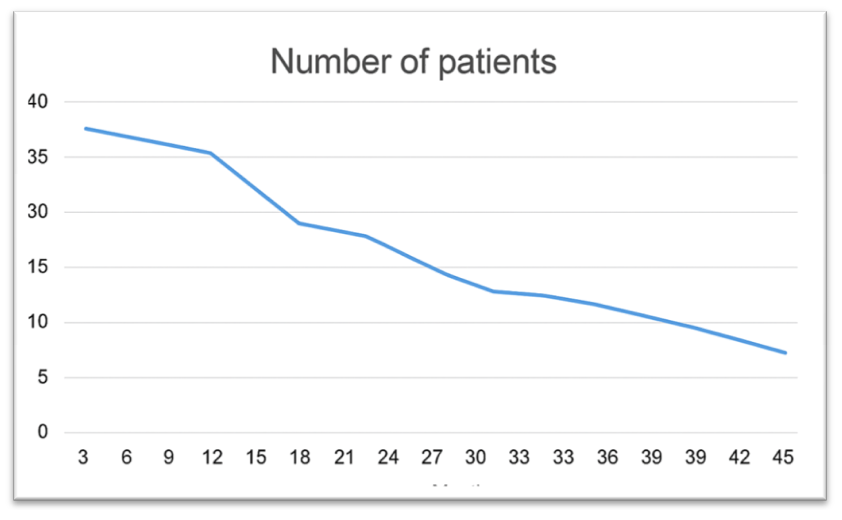 | Figure 1. Long-Term Outcomes of Chemotherapy Combined with Hormonal and Immunotherapy in Breast Cancer Patients with Liver Metastases |
4. Summary
- This study evaluates the effectiveness of a combined immunochemohormonal therapy in 48 patients with luminal A subtype breast cancer and liver metastases. The therapeutic regimen included PIAF polychemotherapy (cisplatin, fluorouracil, doxorubicin), immunotherapy with interferon-α, and hormonal therapy using palbociclib with aromatase inhibitors. After 6 courses, a complete response was observed in 8.3% of patients, partial response in 66.7%, no response in 22.9%, and progression in 2.1%. Despite intensive treatment, 25% of cases developed multidrug resistance. The median survival was 16.5 months; the one-year survival rate was 71.4%, and the four-year survival rate was only 5.7%. The findings suggest that while the combined therapeutic approach has limited efficacy, it provides clinically meaningful benefits in select patients with metastatic breast cancer.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML