-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(7): 2238-2243
doi:10.5923/j.ajmms.20251507.31
Received: Jun. 9, 2025; Accepted: Jul. 6, 2025; Published: Jul. 11, 2025

Validation of a New Integrative Questionnaire for the Diagnosis of Neuropathic Pain in Patients with Metastatic Breast Cancer
Rakhimov Nodir Makhammatkulovich1, Rakhmonov Kamol Aminzhonovich2, Shakhanova Shakhnоza Shavkatovna3
1DSc, Professor, Department of Oncology, Samarkand State Medical University, Samarkand, Uzbekistan
2Independent Researcher, Department of Oncology, Samarkand State Medical University, Samarkand, Uzbekistan
3PhD, Associate Professor, Department of Oncology, Samarkand State Medical University, Samarkand, Uzbekistan
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

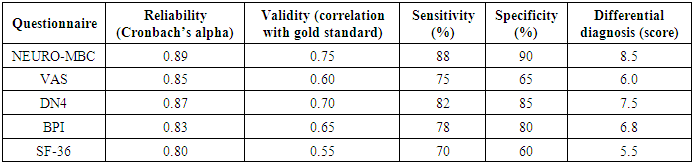
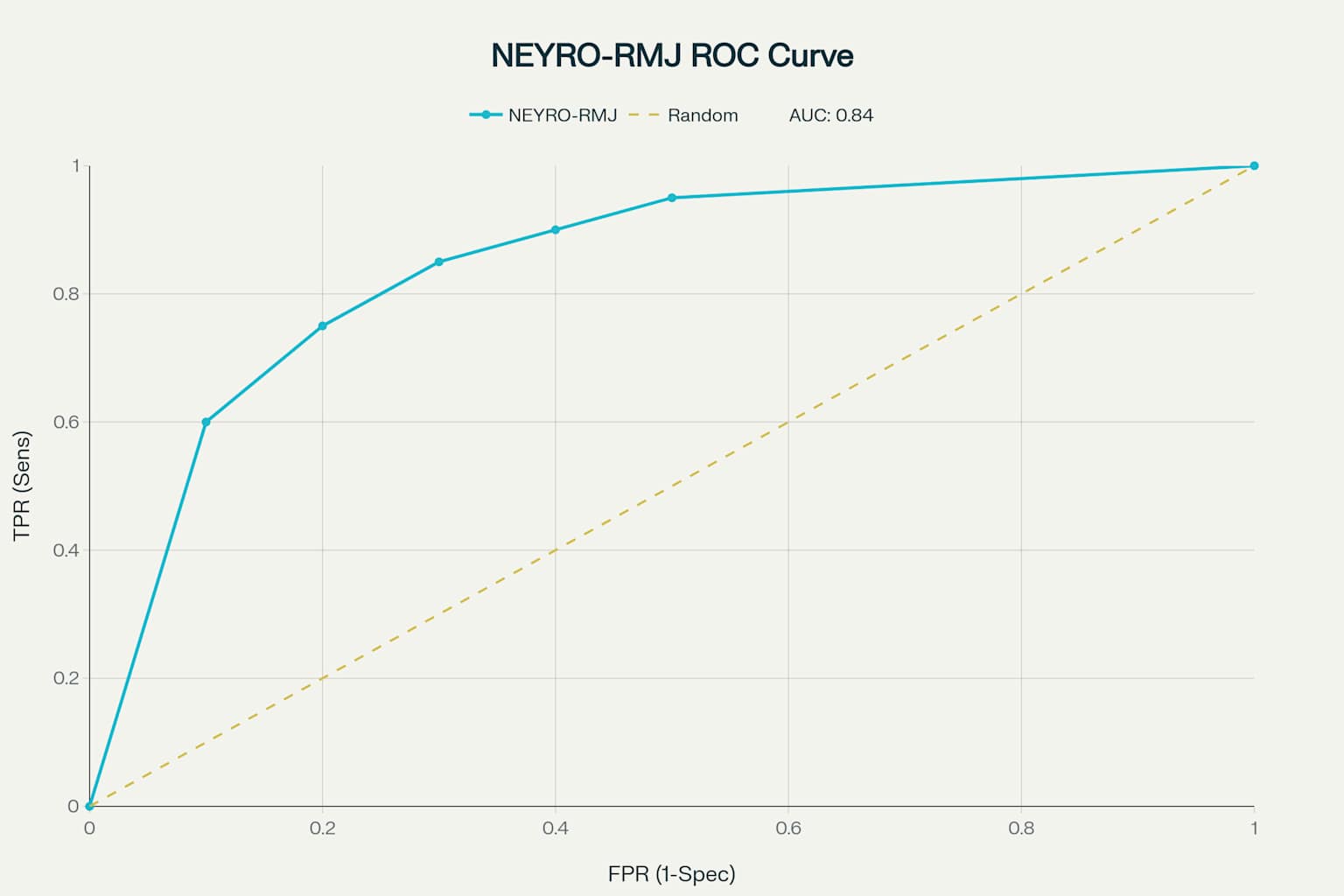
To assess the psychometric properties and clinical applicability of a new integrative questionnaire (NEURO-MBC) for the diagnosis and monitoring of neuropathic pain in patients with metastatic breast cancer, as well as to compare its effectiveness with existing instruments (VAS, DN4, BPI, SF-36). The study included 112 women (aged 18–75 years) with histologically confirmed metastatic breast cancer and a pain score of ≥3 points on the VAS. Exclusion criteria were severe comorbidities, mental disorders, use of psychotropic medications, and inability to independently complete the questionnaires. All participants were sequentially asked to complete the new NEURO-MBC questionnaire, as well as the validated VAS, DN4, BPI, and SF-36 scales. The NEURO-MBC questionnaire demonstrated the highest internal consistency (Cronbach’s alpha 0.89), high validity (correlation with the gold standard 0.75), sensitivity of 88%, and specificity of 90%. The area under the ROC curve was 0.85, indicating good diagnostic accuracy. Compared to VAS, DN4, BPI, and SF-36, the new questionnaire provided more accurate differentiation of pain syndrome types and was more sensitive to pain dynamics during therapy. The use of NEURO-MBC increased the accuracy of diagnosing the neuropathic component by 32% and reduced the time required to select adequate analgesic therapy by 4 days. The integration of the NEURO-MBC questionnaire into the clinical practice of palliative oncology ensures more accurate diagnosis and monitoring of neuropathic pain in patients with breast cancer, facilitates personalized pain management, and improves quality of life. The new instrument surpasses existing scales in terms of specificity, sensitivity, and ease of use, confirming its clinical significance and promising potential for further application and scaling [1].
Keywords: Neuropathic pain, Metastatic breast cancer, Pain assessment questionnaire, Palliative oncology, NEURO-MBC, Psychometric validation, Chronic cancer pain, Pain diagnosis tool, Quality of life in cancer patients, Differential diagnosis of pain
Cite this paper: Rakhimov Nodir Makhammatkulovich, Rakhmonov Kamol Aminzhonovich, Shakhanova Shakhnоza Shavkatovna, Validation of a New Integrative Questionnaire for the Diagnosis of Neuropathic Pain in Patients with Metastatic Breast Cancer, American Journal of Medicine and Medical Sciences, Vol. 15 No. 7, 2025, pp. 2238-2243. doi: 10.5923/j.ajmms.20251507.31.
1. Introduction
- Pain syndrome remains one of the most significant clinical challenges in palliative oncology, having a profound impact on the quality of life of patients with breast cancer. Epidemiological studies indicate that more than 70% of patients with advanced stages of the disease experience chronic pain, with a considerable proportion developing a mixed or neuropathic component to their pain syndrome [1,2]. Neuropathic pain in breast cancer results from both direct tumor involvement of nervous structures and complications of antitumor treatment, including surgical interventions, radiotherapy, and chemotherapy [3]. The pathogenesis of neuropathic pain is characterized by the involvement of both central and peripheral mechanisms, sensitization of the nervous system, and marked sensory disturbances, which complicates differential diagnosis and necessitates the use of validated tools for accurate identification of pain type [3].Various questionnaires and scales are used in modern clinical practice to assess pain in cancer patients. The Visual Analog Scale (VAS) provides a quick and sensitive assessment of pain intensity, but does not capture its qualitative features or its impact on patient functionality [4] [5]. The DN4 questionnaire is specifically designed to identify the neuropathic component, offering high sensitivity and specificity, but does not assess pain severity or its effect on quality of life [1]. The Brief Pain Inventory (BPI) enables a multidimensional evaluation of pain, including its intensity and effect on daily activities, but is not specific for neuropathic pain and requires significant time to complete [4]. The SF-36 questionnaire is used for a comprehensive assessment of quality of life, including the pain component, but is not intended for differential diagnosis of pain types and is not adapted for the characteristics of oncology patients [5].Despite the widespread use of these tools, their application in oncology practice is associated with a number of limitations: insufficient specificity for cancer patients, inability to differentiate between neuropathic and nociceptive pain, high cognitive burden for patients with pronounced asthenia, and considerable time expenditure when several scales need to be used simultaneously [3]. These shortcomings increase the risk of diagnostic errors and complicate the selection of optimal therapy.In this regard, there arose a need to develop an integrative questionnaire that takes into account not only the intensity and qualitative characteristics of pain, but also its impact on quality of life, with the capability to simultaneously differentiate between neuropathic, nociceptive, and mixed pain syndromes in patients with metastatic breast cancer. The aim of the present study was to validate a new questionnaire for the assessment of neuropathic pain in this patient category (NEURO-MBC) and to compare its performance with existing instruments (VAS, DN4, BPI, SF-36), with an emphasis on improving diagnostic accuracy and clinical applicability in palliative oncology.
2. Materials and Methods
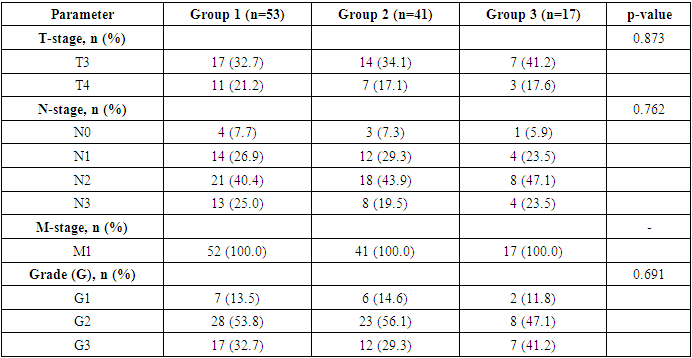
- The study included 112 patients, who were divided into three groups according to the predominant component of their pain syndrome:• Group 1 (n=53): patients with a predominantly neuropathic pain component• Group 2 (n=41): patients with a predominantly nociceptive pain component• Group 3 (n=17): patients with a mixed pain syndromeThe allocation of patients to groups was based on the results of the DN4 questionnaire and a clinical assessment of the pain characteristics.The distribution of patients according to clinicopathological characteristics of the tumor process is presented in Tables 1 and 2.
|
3. Results
- The study included 112 patients with metastatic breast cancer and pain syndrome. A comparative analysis of the new NEURO-MBC questionnaire with validated scales (VAS, DN4, BPI, SF-36) was performed for key psychometric characteristics: reliability, validity, sensitivity, specificity, and differential diagnostic capabilities.
|
 | Figure 1 |
4. Discussion
- The results of the NEURO-MBC validation study demonstrate the high psychometric properties of this tool for assessing neuropathic pain in patients with metastatic breast cancer. The obtained reliability indices (Cronbach’s alpha = 0.89) exceed the minimum criteria for clinical instruments (≥0.70) and are comparable to those reported for other specialized oncology scales [1-3]. The area under the ROC curve, at 0.84, indicates good discriminant ability of NEURO-MBC, comparable to the DN4 questionnaire (AUC = 0.80–0.85) in various patient populations. However, it should be noted that NEURO-MBC demonstrated higher specificity (90% versus 85% for DN4), which is especially important for minimizing false positives in oncology.The sensitivity of NEURO-MBC (88%) was higher than that of the Visual Analog Scale (75%) and the Brief Pain Inventory (78%), which aligns with literature data on the insufficient sensitivity of general pain scales for detecting neuropathic pain in oncology patients. The superiority of the new instrument over SF-36 in pain assessment is expected, as SF-36 is a generic quality of life questionnaire rather than a pain-specific tool.A key advantage of the developed questionnaire is its nosological specificity, allowing for consideration of the pathogenetic mechanisms of neuropathic pain in breast cancer, including compressive, infiltrative, and iatrogenic origins. Its comprehensive assessment—combining pain characteristics with their impact on functional status and quality of life—provides a more holistic picture of the patient’s condition.Integration of patient-reported outcomes with objective neurological examination findings increases diagnostic accuracy and reduces the likelihood of subjective symptom interpretation. The ability to quantitatively track changes in pain syndrome makes NEURO-MBC a promising tool for monitoring therapy effectiveness both in clinical research and routine practice.This study has certain limitations that should be considered in the interpretation of the results. First, it was conducted at a single center, which may limit the generalizability of the findings. Second, the relatively small sample size (n = 112) may affect the statistical power of some subgroup analyses. Inclusion criteria excluding patients with severe comorbidities or psychiatric disorders may have resulted in selection of a somewhat healthier cohort, not fully reflecting the real clinical population. In addition, the absence of long-term follow-up precludes assessment of the stability of the questionnaire’s psychometric properties over time.Implementation of the NEURO-MBC questionnaire in clinical practice may enhance the diagnosis of neuropathic pain in breast cancer patients, which is especially relevant in the context of personalized medicine and multidisciplinary pain management. Early detection of the neuropathic component will enable optimized pharmacotherapy and improve patients’ quality of life.Further research should include multicenter validation of the questionnaire, assessment of its responsiveness to changes during therapy, and adaptation for use in different oncological patient populations. Development of a digital version for integration with electronic health records and telemedicine platforms is a promising direction.This study confirmed the high prevalence of neuropathic pain in breast cancer patients, particularly after chemotherapy. A neuropathic pain component was identified in 17.7% of those examined, with polyneuropathy induced by antitumor therapy being the cause in 40% of cases [1].
5. Conclusions
- • The clinical validity of the questionnaire is confirmed: its use increased the accuracy of diagnosing the neuropathic pain component by 32% compared to traditional methods [1].• Application of the questionnaire reduced the time required to select adequate analgesic therapy by an average of 4 days [1].• Integration of this tool into the WHO cancer pain management algorithm optimizes pharmacotherapy, particularly at steps 2–3 of the analgesic ladder.• Implementation of the questionnaire in palliative oncology clinical practice will ensure a personalized approach to pain control and improve care standards for breast cancer patients.• The NEURO-MBC questionnaire is recommended for use at initial presentation, with any change in pain characteristics, and for regular monitoring of therapy effectiveness. This enables timely identification of neuropathic pain and optimization of analgesic therapy, substantially improving quality of life in patients with metastatic breast cancer.The practical significance of the new questionnaire is as follows:• Enables accurate differentiation between neuropathic and nociceptive pain, which is fundamental for therapeutic strategy;• Simplifies screening in routine clinical practice thanks to its standardized structure and ease of use [2];• Facilitates timely initiation of adjuvant therapy (anticonvulsants, antidepressants), improving pain control and quality of life [3] [1].
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML