-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(7): 2234-2237
doi:10.5923/j.ajmms.20251507.30
Received: Jun. 16, 2025; Accepted: Jul. 7, 2025; Published: Jul. 11, 2025

The Parallel Between the Level of Dysbiosis and the Manifestations of Parkinson's Disease
Rakhimova Malika Mukhamedjanovna1, Sodikova Sayyora Zarifovna2
1DSc., Professor, Departments of Neurology, Tashkent State Dental Institute, Uzbekistan
2PhD Student, Departments of Neurology, Tashkent State Dental Institute, Uzbekistan
Correspondence to: Sodikova Sayyora Zarifovna, PhD Student, Departments of Neurology, Tashkent State Dental Institute, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Dysfunction in the gastrointestinal tract is one of the widespread problems in Parkinson's disease. Recent data increasingly point to a two-way relationship between pathological changes in the gastrointestinal tract, or rather, changes in the composition of the intestinal microbiota and the development of Parkinson's disease, which indicates that more in-depth research is being conducted to correct the composition of the intestinal microbiota, followed by a comparison of the clinical picture of Parkinson's disease. In this article, we will look at the effects of intestinal dysbiosis on the intensity of motor, autonomic, and neuropsychological disorders in Parkinson's disease, and changes after probiotic therapy. We usedUPDRS III, HADS, and GIDS-PD scales and the patient's excrement culture was analyzed to determine the level of dysbiosis before and after an 8-week course of multi-strain probiotic. Improving the composition of the intestinal microbiota led to a significant improvement in the patient's quality of life and reduced the manifestations of Parkinson's disease. Summing up, we can conclude thatprobiotic therapy can be considered as a promising addition to the standard treatment of Parkinson's disease.
Keywords: Parkinson's disease, Intestinal microbiota, Dysbiosis, Probiotic therapy
Cite this paper: Rakhimova Malika Mukhamedjanovna, Sodikova Sayyora Zarifovna, The Parallel Between the Level of Dysbiosis and the Manifestations of Parkinson's Disease, American Journal of Medicine and Medical Sciences, Vol. 15 No. 7, 2025, pp. 2234-2237. doi: 10.5923/j.ajmms.20251507.30.
1. Introduction
- Parkinson's disease (PD), true Parkinsonism or tremor paralysis is a chronic, slowly progressive, degenerative disease of the central nervous system characterized by motor disorders in the form of hypokinesia, muscle rigidity, rest tremor, as well as vegetative, cognitive, affective and other disorders. Although much has been studied in the biomechanism of the development of the disease, the true etiology of sporadic forms of this disease remains unknown. However, in recent years, studies have increasingly shown that the role of the gut, or rather its microbiota, in the progression of the disease is huge and even basic. Constant bidirectional communication between the central nervous system and the gastrointestinal tract - the “microbiota - gut - brain” axis functions in healthy and pathological conditions [1].The gut microbiota is a complex hierarchical ecosystem consisting of 500-1000 species of bacteria, fungi, and viruses [2]. It is a separate "metabolic organ" that not only participates in the digestion of food, but also synthesizes biologically active molecules, modulates immune responses, protects against colonization of pathogenic flora in the intestine, and performs detoxification, anti-carcinogenic, and synthetic functions [3]. Dysbiosis, which occurs in 70-85% of patients with Parkinson's disease and manifests itself 6-7 years before the development of pathognomonic symptoms of PD, is associated with the development of neuroinflammation, impaired neurotransmitter synthesis, and increased BBB permeability [4].A clinical casePatient A. is 65 years old. Diagnosis: Parkinson's disease, akinetic-rigid form. Stage 5 by Hoehn and Yar.Complaints on admission: weakness, lethargy, dizziness, slowness of movement, decreased blood pressure, loss of consciousness in an upright position, memory loss, sweating, stool disorders. Medical history: has been ill for the last 6 years. Prior to this, the blood pressure varied between 170/90-120/80 mmHg. st. Takes Dopadex 50/200 mg irregularly. Defecation was irregular. He suffered from constipation. Was taken Picolax. During the two months prior to admission, there was diarrhea after drinking a large amount of carbonated drinks. Antidiarrheal medications did not help. 4 days lay in the infectious room, after which, diarrhea stopped. Due to the increased frequency of falls when getting up from a horizontal position to a vertical one, which were previously rare, he went to the National Medical Center and was hospitalized in the Neurology department. Objective Status:The patient's condition is severe. Consciousness is clear. The position is forced, lying. Skin is pale and dry. No skin lesions noted. The tongue is coated with a white coating. Peripheral lymph nodes are not palpable.Respiration is rhythmic – 18 breaths per minute. Heart sounds are muffled but rhythmic. Blood pressure: 100/60 mmHg. Pulse rate: 75 bpm.The abdomen is soft, non-tender, with tympanic percussion sounds. Liver and spleen are not enlarged. Kidneys are not palpable.Defecation is spontaneous, with a tendency toward constipation.The patient is unable to stand or walk due to orthostatic hypotension (OH). Blood pressure drops to 80/40 mmHg upon standing.Neurological Status:Consciousness is clear. Speech is slowed. Bilateral anosmia is present. Pupils are equal and reactive to light (D = S). Range of eye movements is full; no nystagmus observed. Face is symmetrical. Tongue is in the midline. Hypomimia is noted. Valle’s points are painless.There is no paresis or paralysis. Deep tendon reflexes (biceps, triceps, patellar, Achilles) are decreased bilaterally (D = S). Muscle strength is preserved. Muscle tone is increased with a plastic (lead-pipe) pattern. The “cogwheel rigidity” phenomenon is positive in both wrists and elbows. Sensation is intact. Babinski reflex is negative. Coordination tests are performed with intention tremor. The patient is unable to maintain Romberg position. The Marinescu–Radovici reflex is positive bilaterally.Higher cortical functions: speech and thinking are slowed (bradyphrenia). Long-term memory is intermittently impaired during episodes of orthostatic hypotension (OH). Depression is present. The patient is vegetatively labile. Pelvic organ function is preserved. No meningeal signs. UPDRS III score: 52 points. HADS score: 8 points. GIDS-PD score: 15 points.Laboratory and instrumental examination data: CBC: Leukocytes: 8.92 × 10⁹/L; Erythrocytes: 3.45 × 10¹²/L; Hemoglobin: 97 g/L; Hematocrit: 28.7%; Platelets: 280 × 10⁹/L; Neutrophils: 7.33 × 10⁹/L; Lymphocytes: 0.77 × 10⁹/L;Monocytes: 0.57 × 10⁹/L; Eosinophils: 0.22 × 10⁹/L;Basophils: 0.03 × 10⁹/L; ESR (Erythrocyte Sedimentation Rate): 49.79 mm/h;Blood Biochemistry: Total protein: 56.1 g/L; Creatinine: 116.9 μmol/L;Urea: 3.13 mmol/L; ALT (Alanine aminotransferase): 56.5 U/L; AST (Aspartate aminotransferase): 68.0 U/L; Potassium: 2.3 mmol/L;Glucose: 2.92 mmol/L.ECG: Rhythm-sinus, regular, heart rate-76 bpm, Electrical axis-deviated to the left, left ventricular hypertrophy, myocardial dystrophy.MSCT angiography: Signs of stenosis of the right vertebral artery.Kinking of the vertebral arteries on both sides.Stool Culture: Bifidobacteria: 10⁴ CFU/g; Lactobacilli: 10⁴ CFU/g;E. coli (lactose-negative): 10⁵ CFU/g. Conclusion: Grade II intestinal dysbiosis.Treatment: 1. Dopadex 50/200 mg 1 tablet once daily at 2000; 2. Tidomed 25/250 mg 1/2 tab. 3 times a day at 800, 1300, 2000 for a long time; 3. Espiro 25 mg 1 tab. once daily in the morning; 4. Carvidilol 6.25 mg 1 tab. once daily; 5. Eleutherococcus 30 drops once daily for 1 month; 6. Probiocare 1 capsule 15 minutes before meals twice daily for 2 months. In this case, it can be concluded that the more the intestinal flora suffers, the more pronounced the manifestations of motor and non-motor, in this case, symptoms of Parkinson's disease. Synucleinopathies, in which pathologically deformed α-synuclein (Levi's bodies) is deposited interneuronally, in Parkinson's disease, in addition to the substantia nigra, pathologically aggregated synuclein also accumulates in postganglionic sympathetic fibers, i.e. orthostatic hypotension is caused by postganglionic noradrenergic denervation of the heart and blood vessels.Purpose of the study: To study the significance of intestinal dysbiosis in the development of clinical signs of Parkinson's disease with its subsequent correction.
2. Materials and Methods of Research
- 40 patients were examined. The subjects were recruited from the NMC of Tashkent, Uzbekistan. Patients independently answered questions on the scales, and also voluntarily provided fecal samples in order to study the degree and nature of dysbiosis in them. Patients answered questions on the GIDS-PD scale to assess upper and lower gastrointestinal symptoms. The severity of constipation was assessed on the Bristol Scale. Motor symptoms were evaluated according to the UPDRS Part III. Psychoemotional disorders were assessed on the HADS scale.
3. Research Results
- The study included 40 patients with an average age of 66.6±7.5, of which 44.4% were men and 65.6% were women. The average age of diagnosis was 54.6±6.5 years, the average duration of the disease was 6.8±1.2 years. According to the forms of the disease, the most common was the mixed form 70%, the akinetic-rigid form 7.5%, and the tremulous form 22.5%.After 8 weeks of probitic therapy, there was a decrease in the severity of dyskinesia on the UPDRS III scale from 36.2 ± 7.8 to 29.7 ± 6.4 (p <0.01). On the GIDS-PD scale, it is possible to note a decrease in dyspeptic phenomena, a decrease in constipation and relief of flatulence with a transition from 17.4 ± 3.1 to 7.2 ± 2.6 (p <0.01), thereby reducing the use of laxatives in patients. The total HADS score before using the multi-strain probiotic was 9.4 ± 2.8 points of anxiety (borderline state), after that, this indicator decreased to 6.7 ± 2.2 points, the level of depression - from 10.1 ± 3.1 to 6.9 ± 2.5 points (p <0.01 for both subscales), which indicates an improvement in the emotional state. In order to detect changes in the imbalance between beneficial, conditionally pathogenic and pathogenic intestinal microflora in patients after probiotic therapy, a bacteriological culture of feces was performed, and the changes were very significant. Before treatment, the majority of patients exhibited grade II and III intestinal dysbiosis, characterized by a reduction in obligate microflora—primarily Bifidobacteria and Lactobacilli—as well as an increase in conditionally pathogenic flora, mainly lactose-negative E. coli.Prior to therapy, Bifidobacteria and Lactobacilli concentrations ranged from 10⁵ to 10³ CFU/g, while lactose-negative E. coli ranged from 10⁵ to 10⁶ CFU/g.Following a course of probiotic therapy, Bifidobacteria and Lactobacilli concentrations increased to 10⁸–10⁹ CFU/g, and the levels of conditionally pathogenic strains decreased to within or close to the normal range.
4. Conclusions
- Thus, the use of probiotic therapy for a duration of 8 weeks or longer led to improvements in clinical scale scores, reduction of motor symptoms, correction of psycho-emotional status, relief of defecation difficulties, and normalization of intestinal microbiota composition.It is likely that these improvements are mediated by enhanced gastrointestinal function, reduced inflammatory and endotoxin-related effects of pathogenic flora, increased bioavailability of levodopa, and decreased neuroinflammation through the microbiota–gut–brain axis (Figure 1).
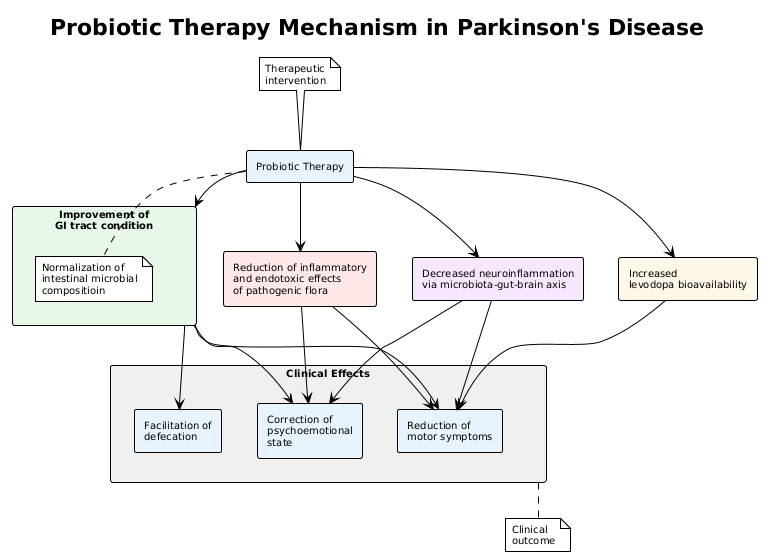 | Figure 1. Probiotic Therapy Mechanism In Parkinson’s Disease |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML