-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(7): 2228-2233
doi:10.5923/j.ajmms.20251507.29
Received: Jun. 12, 2025; Accepted: Jul. 6, 2025; Published: Jul. 11, 2025

The Impact of Thyroid Diseases on the Reproductive Health of Adolescent Girls: Literature Review
Amina Kalandarova
Medical Institute of Karakalpakstan, Nukus, Uzbekistan
Correspondence to: Amina Kalandarova, Medical Institute of Karakalpakstan, Nukus, Uzbekistan.
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Iodine deficiency diseases are among the most common non-communicable human diseases. It is estimated that more than 1.5 billion people (28.9% of the global population) are at increased risk of insufficient iodine intake, and 655 million people already have an enlarged thyroid gland (endemic goitre). The spectrum of iodine deficiency disorders is wide. In addition to the most common manifestation of iodine deficiency, non-toxic goitre, iodine deficiency can lead to impaired reproductive function, delayed puberty and physical development, and delayed intellectual development, up to the formation of severe forms of cretinism. It can also serve as a background for the growth of somatic pathology. The most unfavourable consequences occur during the early stages of bodily development, from the intrauterine period to puberty. Thus, most authors point to an increase in the frequency of endemic goitre in children with age, reaching a maximum during puberty, which is associated with stress on the neuroendocrine system due to active growth and sexual development. The aim is to assess the impact of thyroid diseases on the reproductive system of women and adolescent girls. A systematic literature review was conducted using four international databases (PubMed, Web of Science, Scopus and Google Scholar), resulting in a total of 50 publications.
Keywords: Adolescents, Reproductive system, Girls, Graves' disease, Diffuse toxic goiter, Hypothyroidism, Hyperthyroidism, Thyroid disease
Cite this paper: Amina Kalandarova, The Impact of Thyroid Diseases on the Reproductive Health of Adolescent Girls: Literature Review, American Journal of Medicine and Medical Sciences, Vol. 15 No. 7, 2025, pp. 2228-2233. doi: 10.5923/j.ajmms.20251507.29.
1. Introduction
- Currently, the problem of iodine deficiency is global. This is due to the prevalence of iodine deficiency diseases (IDD) in many countries and the large number of people involved in it. The spectrum of iodine deficiency diseases is wide and in addition to the most common manifestation of iodine deficiency - non-toxic goiter, iodine deficiency can lead to impaired reproductive function and puberty, to delayed physical development, as well as to delayed intellect up to the formation of severe forms of cretinism and can serve as a background for an increase in somatic pathology [7]. Serious problems in the state of somatic and reproductive health of an individual family and its offspring arise with prolonged living in conditions of iodine deficiency: thyroid disease, mental and physical retardation, neonatal goiter, infertility, miscarriages, complicated pregnancy and childbirth, stillbirths, congenital anomalies [18]. The most severe consequences of iodine deficiency for the body (iodine deficiency diseases) are observed during critical periods of development: intrauterine, puberty, pregnancy [19]. According to the authors Hollowell J.G. et al. thyroid dysfunction is a common endocrine disorder. In the US National Health and Nutrition Examination Survey, the prevalence of hypothyroidism was 4.6% (0.3 overt and 4.3% subclinical) and the prevalence of hyperthyroidism 1.3% (0.5 overt and 0.7% subclinical) in people without known thyroid disease or a family history of thyroid disease [1,3].In their study, Unnikrishnan AG et al report that a study from eight major Indian cities have shown the prevalence of hypothyroidism in the urban India is 10.95% in which 3.47% were previously undetected, and 7.48% were self-reported cases.Krassas GE at al found that hypothyroidism causes an increase in the level of thyrotropin-releasing hormone (TRH), which in turn stimulates the secretion of thyroid-stimulating hormone (TSH) and prolactin (PRL), and PRL suppresses the synthesis and secretion of gonadotropins. Several studies have also confirmed abnormal menstrual cycles in overt hypothyroidism [5,6,7].Thus, the aim of this study is to systematize modern scientific knowledge about the relationship between environmental factors, iodine deficiency and the development of disorders in the reproductive system of adolescent girls in order to develop effective preventive strategies in the context of increasing environmental threats, as well as to assess new risk factors for reduced fertility and the possibilities of their prevention. To achieve this goal, the study set the following objectives: to identify various pathological conditions of the thyroid gland that have the greatest impact on the development of disorders in the reproductive system of adolescent girls based on modern epidemiological studies; to assess the effectiveness of modern technological, pharmacological and socio-economic approaches to the prevention of environmentally conditioned diseases at the individual, local and population levels in order to determine optimal strategies for their implementation.
2. Materials and Methods
- This study was conducted as a systematic literature review aimed at identifying patterns of impact of various thyroid pathological conditions on the health of expectant mothers - adolescent girls. Relevant publications were retrieved from the electronic databases PubMed, Web of Science, Scopus and Google Scholar. A combination of keywords and their variations were used, including: Adolescents, reproductive system, girls, Graves' disease, diffuse toxic goiter, hypothyroidism, hyperthyroidism, thyroid disease. All retrieved records were initially systematized and filtered using EndNote (version 20.2) to eliminate duplicates and compile the main study corpus. EndNote was used for centralized reference management and subsequent systematic review. A local reference library was created into which records from each database were imported in RIS or XML formats. Publications were sorted by keywords, journal titles, and first author names to facilitate thematic grouping and analysis of sources. To improve the accuracy of filtering, the advanced search function was used to apply multiple criteria simultaneously (e.g., time range, specific scientific terms), thereby excluding irrelevant records. Tags and personalized notes were assigned during further processing to indicate the potential value of a source in relation to specific aspects of the study. All article metadata was stored in a structured format to allow rapid access to full texts via embedded hyperlinks. A “Smart Groups” function was also used to automatically update source collections whenever new records matching predefined search criteria were added to the library.The process of selecting sources followed the principles of systematic reporting according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [9]. The corresponding scheme is presented in Figure 1.
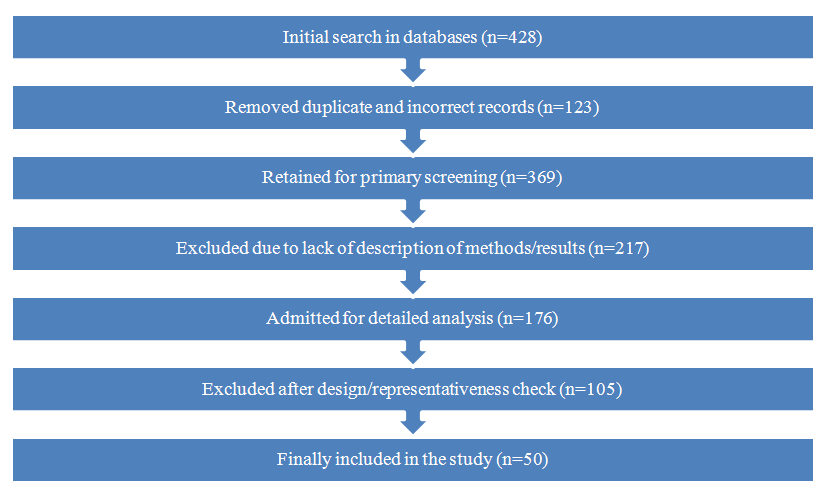 | Figure 1. PRISMA diagram for source selection |
3. Results and Discussion
- Thyroid hormones control the metabolism of all nucleated cells and are therefore vital for various processes, including gametogenesis, fertilization, embryogenesis, implantation, fetal development, and growth in utero [1,2]. Hyperthyroidism is a pathological state characterized by the excessive production of thyroid hormones, which leads to elevated serum levels of thyroxine (T4) and triiodothyronine (T3), as well as diminished serum levels of thyroid-stimulating hormone [3]. The various etiological and pathological mechanisms leading to hyperthyroidism, as well as the different treatment modalities, can negatively impact male and female fertility, conception, fetal and maternal well-being, and the perinatal and adult lives of fetuses exposed in utero [2,4,5]. Previously, hyperthyroidism was reported to have a prevalence of less than 1%, primarily affecting middle-aged and elderly populations [6]. Recent epidemiological surveys suggest prevalence rates of up to 1.6% in populations recovering from endemic iodine deficiency following universal salt iodization, primarily presenting as toxic thyroid nodules not only among the elderly, but also among persons aged 20–49 years [7,8,9]. Pedersen et al. reported a 160% increase in the prevalence of hyperthyroidism in the 20–39 age group in Denmark following food fortification with iodine. In Ghana, Sarfo-Kantanka et al. [8] reported a 213-538% increase in thyroid disease-related hospital admissions 20 years after universal salt iodization. Toxic nodular goiter was the second most common presentation, affecting mainly women (with a female-to-male ratio of 8.3:1) in the 27–42 age range. Although transient, this increase in hyperthyroidism following improved access to iodine nutrition can last up to 10 years [10,11]. This is followed by a decline in hyperthyroidism prevalence in countries that attain and maintain optimal iodine nutrition [7,9,12]. It is not clear whether excessive iodine intake in formerly iodine deficiency endemic populations, or recurrent exposure to iodine deficiency in pregnancy like, has been reported in some European countries [13,14] can lead to prolong the ‘transient increase’ in hyperthyroidism secondary to iodine fortification. One study from south China reported that pregnancy not only predisposes to hypertrophy of pre-existing nodules, also but to the formation of new nodules with biochemical milieu close to subclinical hyperthyroidism [15].X. Hu et al. (2022) conducted a meta-analysis of studies to assess the risk of polycystic ovary syndrome (PCOS) in girls with Hashimoto's thyroiditis. Their meta-analysis of two studies showed that patients with autoimmune thyroiditis are at a higher risk of developing PCOS than healthy girls [25,26].The pathogenesis of PCOS is currently unclear. It has been suggested that it is associated with genetic, metabolic, hormonal, and immune factors [26,27]. Three genetic polymorphisms that play a role in both PCOS and Hashimoto's thyroiditis have been described. These are the fibrillin 3 gene polymorphism, the gonadotropin-releasing hormone receptor gene polymorphism, and the CYP1B1 polymorphism, which is responsible for estradiol hydroxylation. The fibrillin gene regulates the activity of transforming growth factor (TGF)-beta, affecting T regulatory cells. Decreased TGF-beta and T-regulatory cell activity contributes to the development of autoimmune diseases and serves as a predisposing factor for the co-development of PCOS and autoimmune thyroiditis.Thyroid autoimmunity and fertilityPublications assessing the prevalence of thyroid autoantibodies in infertile women have reported divergent results, which may be explained by heterogeneous study designs (retrospective, prospective, and cross-sectional), different populations studied (various ages of subjects and causes of infertility), and different assays used to determine thyroid antibodies. It is noteworthy that, in a recent prospective study carried out among 1,054 fertile women with a history of one or two prior pregnancy losses, there was no difference in the pregnancy rates between 154 women with TAI and 900 women without TAI (74% vs. 72.2%, respectively, p = 0.64) [34]. However, summarized data have shown an association between TAI and decreased fertility. In a review study of women with various causes of infertility, Poppe et al. demonstrated a significantly higher incidence of TAI [risk ratio (RR): 2.1, p < 0.0001] [35]. In a meta-analysis by van den Boogaard et al. [36], the presence of thyroid antibodies was associated with a higher risk of unexplained subfertility [odds ratio (OR): 1.5, 95% confidence interval (95% CI): 1.1–2.0]. An elevated prevalence of TAI might be especially concerning in some particular causes of infertility: polycystic ovary syndrome (PCOS) (26.9%), idiopathic infertility, and endometriosis (25%) [37-40]. In a 2018 meta-analysis of 13 cross-sectional and case–control studies evaluating a total of 1,210 women with PCOS and 987 healthy controls, Romitti et al. [41] found a significant association between PCOS and TAI (OR: 3.27, 95% CI: 2.32–4.63). A predisposing factor for the co-occurrence of PCOS and TAI is a polymorphism in the fibrillin gene, which regulates transforming growth factor-β (TGF-β) activity, which, in turn, affects Treg cells. Reduced TGF-β and Treg activity promotes the development of autoimmune diseases. Another predisposing factor is the high estrogen/progesterone ratio found in women with PCOS and vitamin D deficiency [42]. TAI leading to (sub) hypothyroidism may negatively affect metabolic performance, higher triglycerides, and free testosterone in women with PCOS [43]. Some studies have found TAI in 25%–46% of women with endometriosis attending infertility clinics [29,40.44], although this observation has not been confirmed by other observations [41,45]. The pathophysiology of endometriosis is complex and still unclear, but the condition is associated with a variety of inflammatory and immunological phenomena, such as the presence of autoantibodies to endometrial antigens (including aLN-1), complement deposits, apoptosis, a decline in NK cell concentration, and cytotoxic effects on the endometrium [46,47]. Reciprocally, thyroid antibodies can affect the human endometrium, including ectopic endometrium, as all of the transcripts involved in thyroxin synthesis have been found in the endometrium, including TPO and Tg [1]. Several studies have pointed out a possible association between TAI and diminished ovarian reserve or premature ovarian insufficiency (POI). From 4% to 30% of POI cases are autoimmune in origin [23]; in a recent meta-analysis, the authors confirmed a higher frequency of TPOAb positivity in this group of patients (OR: 2.26, 95% CI: 1.31–3.92, p = 0.004), but not of TgAb positivity [48]. After pooling data from 30 studies published between 1997 and 2021, they concluded that women of reproductive age with Hashimoto’s thyroiditis (hypothyroid and euthyroid) have lower concentrations of anti-Müllerian hormone and antral follicle count. Unfortunately, they did not perform a subanalysis in a group of euthyroid women. In a recent publication [49] not included in the abovementioned meta-analysis, retrospective research of 4,302 euthyroid women proved that TAI was associated with POI only in the group with TSH > 2.5 µIU/ml but not in those with TSH ≤ 2.5 µIU/ml. These facts indicate that there may be a role for relative thyroid hormone insufficiency acting together with thyroid autoimmunity in the process of ovarian damage. The European Society of Human Reproduction and Embryology recommends testing for TPOAb in women with POI [50].Nodular goiter, a condition characterised by thyroid nodules, can affect a woman's reproductive system due to its impact on thyroid hormone levels. Disruptions to thyroid function, such as hypothyroidism (producing too little thyroid hormone) or hyperthyroidism (producing too much thyroid hormone), can result in menstrual irregularities, infertility and other reproductive problems.Here's a more detailed look at the potential impact:1. Menstrual irregularities and amenorrhoea:• Hypothyroidism:Can cause irregular periods, light or heavy periods, or even complete cessation of periods (amenorrhoea).• Hyperthyroidism:This can also lead to menstrual irregularities, including heavy, prolonged or irregular bleeding.• Autoimmune thyroiditis:If the thyroid disease is autoimmune, it may also affect other glands, including the ovaries, further impacting reproductive function.2. Infertility:• Hypothyroidism:Low thyroid hormone levels can interfere with ovulation, making it difficult for a woman to conceive.• Hyperthyroidism:Similarly, high thyroid hormone levels can disrupt ovulation and impact fertility.• Underlying causes:The underlying causes of hypothyroidism or hyperthyroidism, such as autoimmune disorders, may also impair fertility.3. Pregnancy complications:• Both hypo- and hyperthyroidism:These conditions can increase the risk of complications during pregnancy, such as miscarriage, preterm birth and low birth weight.• Thyroid nodules:Some studies suggest that thyroid nodules may grow during pregnancy, which could impact thyroid function and increase the risk of complications.4. Gynaecological conditions:• Hypothyroidism:This can be associated with conditions such as premature ovarian insufficiency and polycystic ovary syndrome (PCOS), which can further impact reproductive health.Hyperthyroidism:This can also affect ovarian function and may be associated with certain gynaecological issues. 5. Other reproductive issues:• Thyroid hormonesare crucial for the development and function of the ovaries, uterus and placenta.Disruptions in thyroid hormone levels can affect various aspects of reproductive health, including the endometrium (the lining of the uterus) and the implantation process.In summary, thyroid issues, including nodular goiter, can significantly impact a girl's reproductive health due to their influence on hormone balance and the overall functioning of the reproductive system.
4. Conclusions
- Although hyperthyroidism has a limited impact on fertility, if left undiagnosed or not effectively controlled, it can greatly reduce fertility for both fertile women and those undergoing fertility treatment, due to early pregnancy losses and preterm delivery, which may be caused by maternal complications. The transient increase in hyperthyroidism secondary to iodine fortification is associated with a higher incidence of non-toxic and toxic thyroid nodules among women in the second half of their reproductive years, and with Graves' disease among younger women. This may increase not only the incidence of perinatal, congenital, and behavioural complications associated with in utero exposure to ATD and TRabs, but also maternal mental health and cardiovascular complications. Further studies are needed to elucidate the epidemiology of hyperthyroidism and other thyroid diseases, especially in populations with recurrent iodine deficiency or excess alongside iodine fortification, as well as increased vigilance during prenatal and postnatal care. Poorly controlled hyperthyroidism during pregnancy is associated with maternal morbidity and mortality. Long-term follow-up of children born to mothers with hyperthyroidism is crucial, as these children may exhibit neurocognitive disorders.Our study demonstrated that hypothyroidism in women causes menstrual irregularities, primarily oligomenorrhoea. Hypothyroidism is associated with hyperprolactinemia and decreased serum levels of E2, T and Gn, which increase after euthyroidism is achieved.Thyroid autoimmunity is not only associated with thyroid hormone deficiency, but also with a broader spectrum of immune disturbances that can lead to decreased fertility and an increased risk of miscarriage. A better understanding of the pathophysiological pathways of TAI is essential for the development of successful future therapies. As levothyroxine supplementation has been found to be ineffective in preventing adverse pregnancy outcomes, future research should probably focus on restoring immune balance.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML