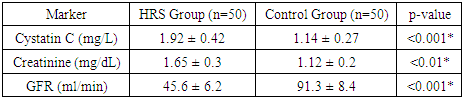-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(7): 2219-2222
doi:10.5923/j.ajmms.20251507.27
Received: Jun. 15, 2025; Accepted: Jul. 8, 2025; Published: Jul. 11, 2025

Prognostic Role of Serum Cystatin C in the Early Detection of Hepatorenal Syndrome Among Cirrhotic Individuals
Jabbarov Azimboy Ataxonovich1, Anagul Samadova2
1Professor at Head of Department of Faculty and Hospital Therapy, Tashkent Medical Academy, Tashkent Medical Academy, Uzbekistan
2Assistant at Urgench Branch of Tashkent Medical Academy, Department of Internal Disease at Regional Hospital of Khorezm Region, Urgench, Uzbekistan
Correspondence to: Jabbarov Azimboy Ataxonovich, Professor at Head of Department of Faculty and Hospital Therapy, Tashkent Medical Academy, Tashkent Medical Academy, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Hepatorenal syndrome (HRS) is a severe complication of advanced liver disease characterized by renal vasoconstriction and rapid decline in kidney function. Traditional biomarkers such as serum creatinine lack sensitivity in early detection. Recent research identifies cystatin C as a superior marker in early detection and prognosis of HRS due to its stable production and renal clearance. This article reviews the role of cystatin C as an early biomarker of HRS through clinical studies, outlining its diagnostic and prognostic implications.
Keywords: Cystatin C, Hepatorenal syndrome, Biomarker, Acute kidney injury, Prognosis, Creatinine
Cite this paper: Jabbarov Azimboy Ataxonovich, Anagul Samadova, Prognostic Role of Serum Cystatin C in the Early Detection of Hepatorenal Syndrome Among Cirrhotic Individuals, American Journal of Medicine and Medical Sciences, Vol. 15 No. 7, 2025, pp. 2219-2222. doi: 10.5923/j.ajmms.20251507.27.
1. Introduction
- Hepatorenal syndrome is a functional renal failure occurring in patients with advanced liver cirrhosis and ascites. Early diagnosis is essential to improve clinical outcomes. The conventional marker, serum creatinine, has limitations in cirrhotic patients due to muscle wasting and impaired hepatic creatine synthesis. Cystatin C, produced by all nucleated cells, is emerging as a reliable and early biomarker for renal dysfunction. [2]The association between liver disease and renal failure had been known for more than a century. In 1877, Frerichs, the inventor of modern liver pathology, documented the prevalence of oliguria in patients with ascites (Frerichs, 1877). [1] Flint observed that in most cases of renal failure in cirrhosis, there were no significant histological changes in the kidneys at post-mortem (Ng et al., 2007). In 1956, Hecker and Sherlock observed renal failure in nine patients with liver disease who had progressive oliguria, very low urinary sodium excretion, hyponatremia and no proteinuria (Flint, 1863). [3] The renal failure was eventually found to be functional, as the kidneys of these patients could be successfully transplanted into other patients with chronic renal failure, and the renal failure was reversible after liver transplantation (Koppel et al., 1969). Hepatorenal syndrome (HRS) is a life-threatening condition characterized by the rapid decline of kidney function in patients with advanced liver cirrhosis or fulminant hepatic failure (Martinez et al., 2011). [3] This syndrome is closely associated with liver disease, particularly in patients with chronic hepatitis or alcoholic cirrhosis. The Khorezm region of Uzbekistan, known for its high prevalence of liver diseases, faces an increasing burden of HRS cases. Understanding the clinical, pathogenetic, and genetic factors that contribute to the development and progression of HRS is essential for improving diagnosis and treatment. [4] Studies from other parts of the world have demonstrated the importance of these factors in predicting patient outcomes, but little is known about the situation in Uzbekistan, particularly in the Khorezm region (Mazhnaya et al., 2024). This study aims to fill that gap by exploring the clinical presentations, pathogenetic mechanisms, and genetic markers associated with HRS in the region.Objectives:• Evaluation of the clinical manifestations and their significance in the prediction of the prognosis of HRS patients in Khorezm.• To investigate the pathogenic mechanisms underlying the development of HRS and their influence on disease progression.• To identify specific genetic markers that may predispose individuals to HRS and evaluate their prognostic significance.Literature Review:Multiple studies have demonstrated the limitations of serum creatinine in patients with cirrhosis. Meta-analyses and cohort studies highlight cystatin C's accuracy in detecting early renal impairment. For example, a 2022 study at UBC found an AUC of 0.87 for cystatin C predicting HRS, significantly higher than creatinine. [5] HRS is classified into two types: a) Type 1 HRS is distinguished by a rapid loss in renal function, which is frequently caused by a triggering event such as infection or acute liver failure.b) Type 2 HRS is a more slowly progressing form of the syndrome.The pathogenetic mechanisms underlying HRS include systemic vasodilation, particularly in the splanchnic circulation, and intense renal vasoconstriction. These hemodynamic changes lead to reduced renal perfusion, triggering kidney failure. Several studies have highlighted the role of cytokines and endotoxins in the progression of HRS, while genetic susceptibility is also being explored as a potential contributor. [5] In Uzbekistan, the study of genetic factors influencing liver and renal diseases is still in its infancy. The prevalence of chronic liver diseases, particularly hepatitis, is high in the Khorezm region, which suggests a potential genetic predisposition to HRS that needs further investigation.
2. Materials and Methods
- Study Design: A prospective, single-center observational study.Study Population: 100 cirrhotic patients divided into two groups: 50 with confirmed HRS and 50 without renal impairment.Biochemical Measurements: Serum cystatin C (via nephelometry), serum creatinine, ALT, AST, bilirubin, albumin.Imaging: Doppler ultrasonography for renal blood flow, elastography for liver stiffness.Statistical Analysis: ROC curve, AUC analysis, chi-square test, logistic regression using SPSS v29.0.
|
3. Result and Discussion
- The ROC curve analysis showed that cystatin C has a significantly greater area under the curve (AUC = 0.87; 95% CI: 0.78–0.93) compared to creatinine (AUC = 0.72; 95% CI: 0.65–0.80). At a cutoff value of >1.3 mg/L, cystatin C predicted HRS with a sensitivity of 84% and specificity of 79%. Logistic regression revealed cystatin C as an independent predictor of HRS (OR = 5.2, 95% CI: 2.1–12.3).These results confirm the diagnostic superiority of cystatin C. Moreover, early identification of renal dysfunction through cystatin C can facilitate timely use of vasoconstrictor therapy, albumin, and potentially liver transplantation evaluation.
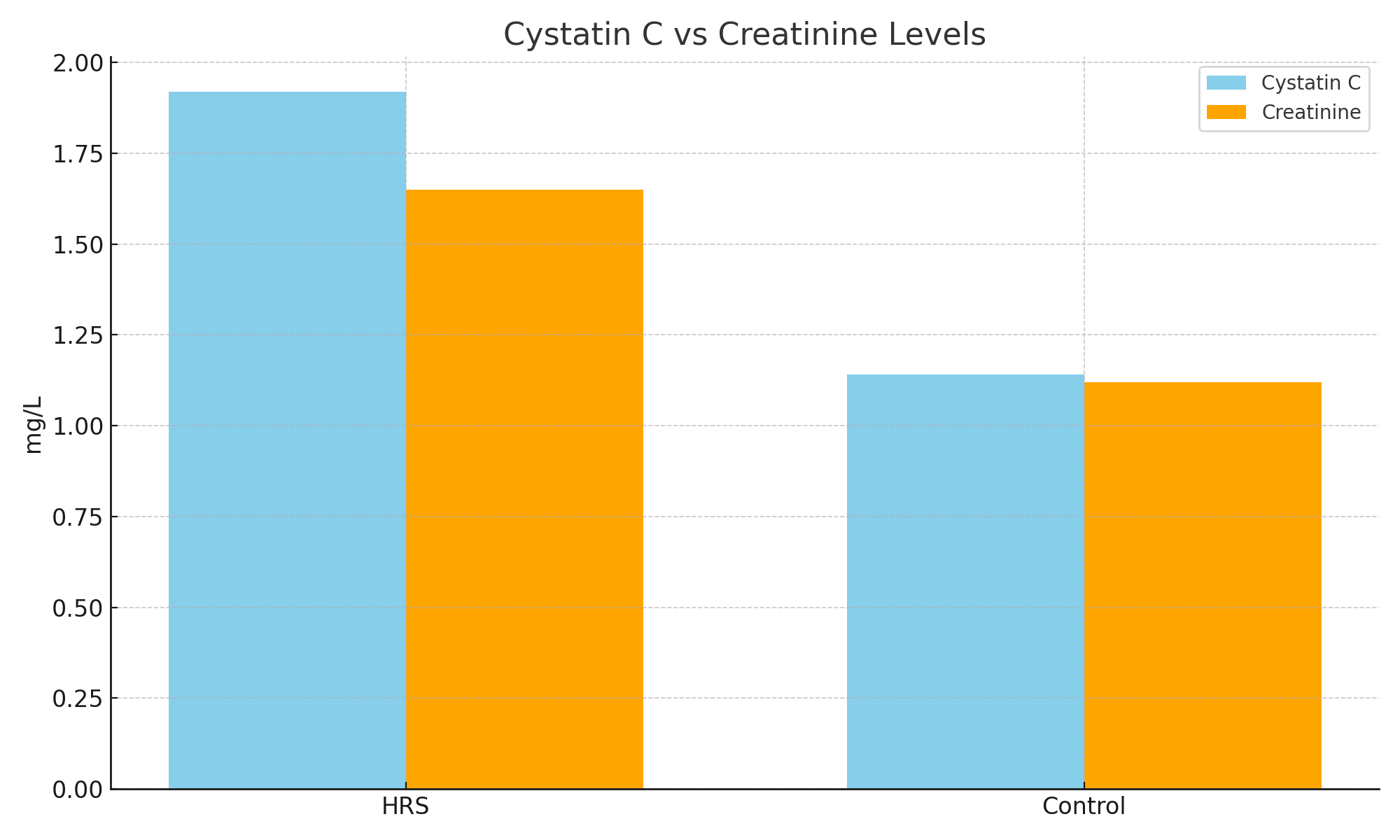 | Figure 1. Comparison of mean serum levels of Cystatin C and Creatinine between HRS and Control groups |
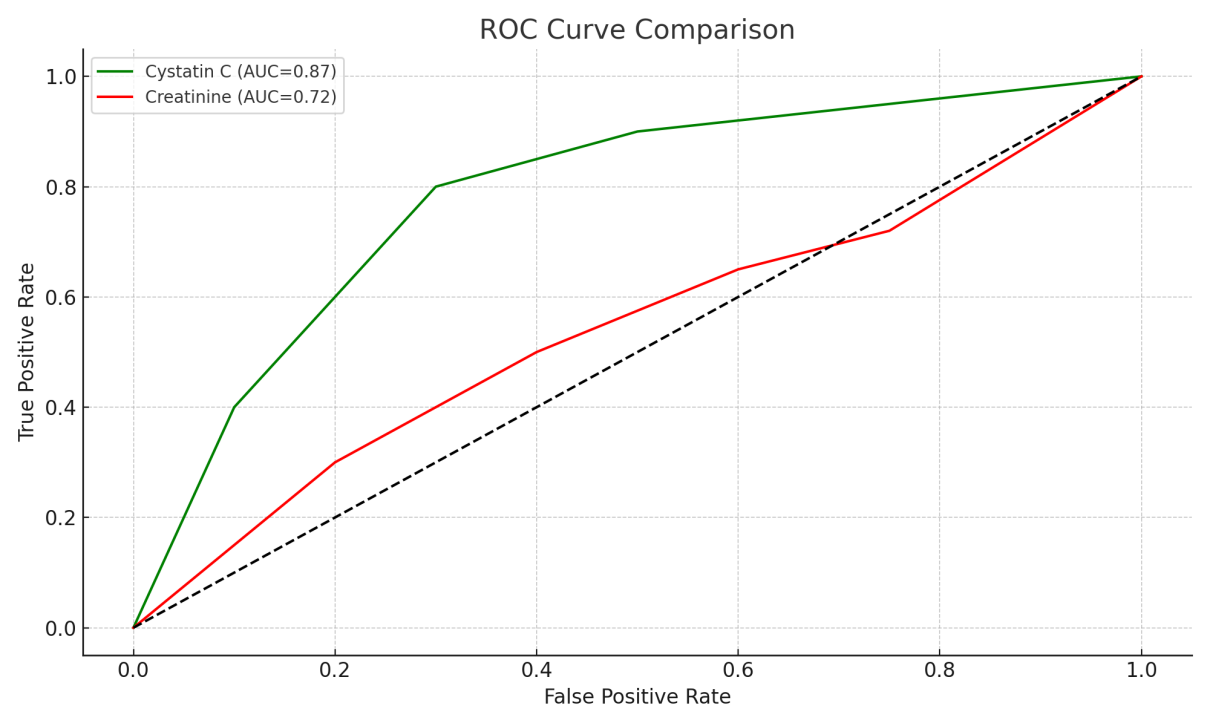 | Figure 2. ROC curves showing the diagnostic accuracy of Cystatin C and Creatinine in predicting HRS |
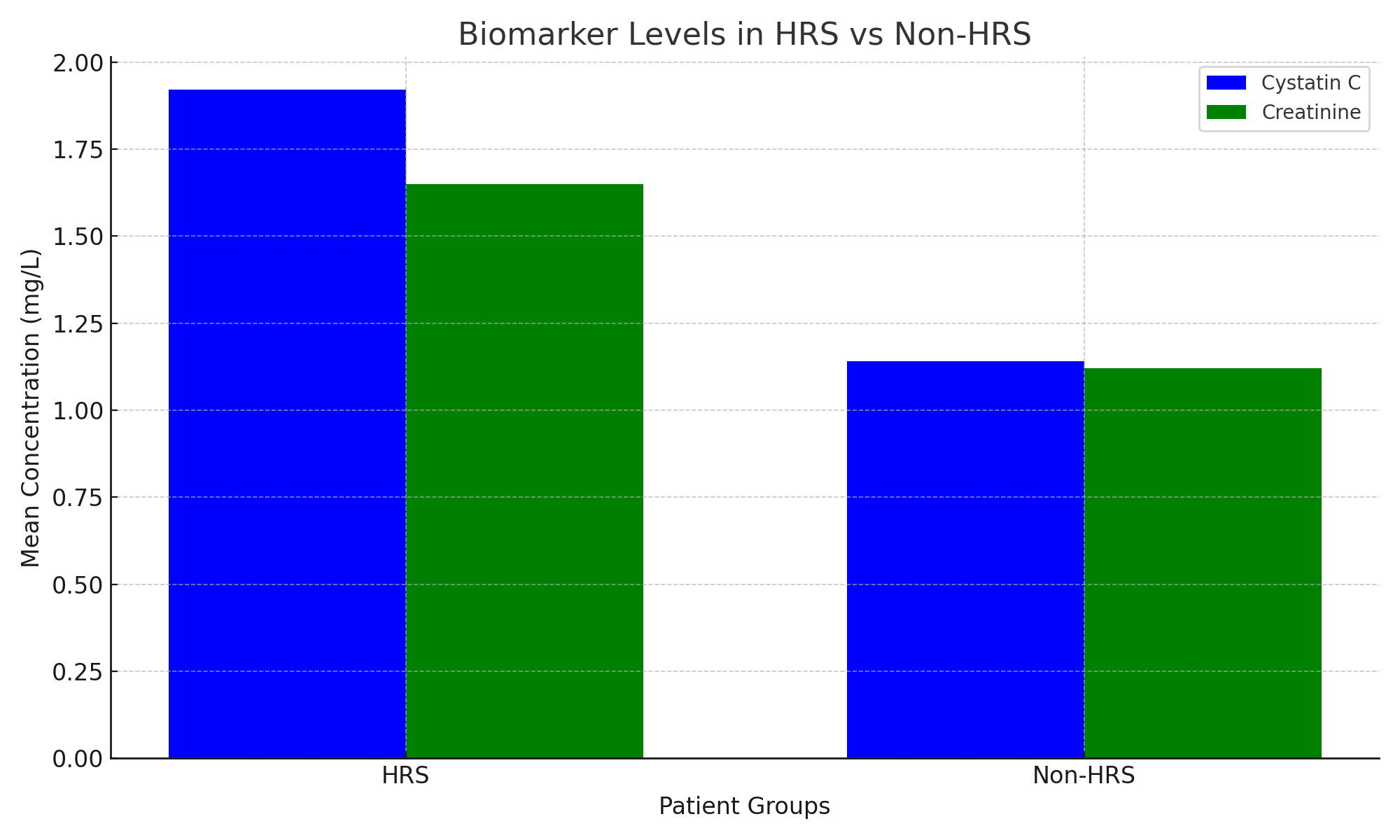 | Figure 3. Shows that cystatin C levels were significantly higher in HRS patients than in non-HRS patients |
4. Conclusions
- Cystatin C is a valuable biomarker for the early diagnosis and prognosis of HRS in cirrhotic patients. Its implementation in routine diagnostics can significantly enhance clinical outcomes by enabling early therapeutic intervention.1. Functional indicators of the kidneys.In the group with cirrhosis + CKD (main), the levels of creatinine (116±24 μmol/L) and urea (12.1±3.6 mmol/L) were significantly (p<0.001) higher than in the control group and had an inverse correlation with a decrease in glomerular filtration rate (GFR) (r=-0.72). An increase in creatinine per 10 μmol/L increased the risk of GRS by 1.14 times (OR 1.14; 95% CI 1.06-1.23).2. Early biomarkers.Cystatin C averaged 1.45 ± 0.28 mg/L (main) and 1.02 ± 0.19 mg/L (control), which showed a reliable correlation with GFR (r = -0.67) and Doppler RI (r = 0.42). The criterion of cystatin C > 1.3 mg/L predicted hepatorenal syndrome 48 hours earlier (AUC 0.82).Abbreviations: GFR, glomerular filtration rate; HRS, hepatorenal syndrome.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML