-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(7): 2175-2178
doi:10.5923/j.ajmms.20251507.18
Received: Jun. 9, 2025; Accepted: Jul. 3, 2025; Published: Jul. 11, 2025

The Role of Inflammatory Cytokines and Growth Factors in the Carcinogenesis of the Oral Mucosa in Patients with Combined Leukoplakia and Type 2 Diabetes Mellitus
Rasulova Nargiza Azamatovna, Khabibova Nazira Nasulloyevna
Bukhara State Medical Institute, Bukhara, Uzbekistan
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The study assesses the pathogenetic influence of inflammatory cytokines and growth factors on the malignant transformation of the oral mucosa in patients with combined leukoplakia and type 2 diabetes mellitus. Chronic metabolic destabilization in diabetes contributes to microvascular impairment and sustained oxidative stress, creating a permissive background for epithelial dysplasia. An increased expression of TNF-α, IL-1β, IL-6, IL-17, IL-23, and vascular endothelial growth factor is observed in dysplastic lesions, with a parallel elevation of MMP-8, MMP-9, and MMP-13 levels. These mediators are associated with degradation of extracellular matrix components, loss of epithelial integrity, and stimulation of angiogenesis. The microbial colonization by Fusobacterium nucleatum, Prevotella spp., and Porphyromonas gingivalis potentiates oncogenic signaling through activation of NF-κB, MAPK, and β-catenin pathways. The interplay between cytokine-induced inflammation and bacterial virulence contributes to cellular proliferation, DNA damage, and resistance to apoptosis in the precancerous epithelium. These findings reflect a synergistic mechanism of microenvironmental carcinogenesis modulated by both immune and metabolic factors in the diabetic oral cavity.
Keywords: Oral leukoplakia, Type 2 diabetes mellitus, Chronic inflammation, TNF-α, IL-1β, IL-6, IL-17, IL-23, MMP-9, VEGF, Fusobacterium nucleatum, Prevotella spp., Porphyromonas gingivalis, NF-κB, MAPK, β-catenin, Epithelial dysplasia, Metabolic microenvironment, Oral carcinogenesis, Proinflammatory mediators
Cite this paper: Rasulova Nargiza Azamatovna, Khabibova Nazira Nasulloyevna, The Role of Inflammatory Cytokines and Growth Factors in the Carcinogenesis of the Oral Mucosa in Patients with Combined Leukoplakia and Type 2 Diabetes Mellitus, American Journal of Medicine and Medical Sciences, Vol. 15 No. 7, 2025, pp. 2175-2178. doi: 10.5923/j.ajmms.20251507.18.
1. Introduction
- Carcinogenic transformation of the oral mucosa in the context of leukoplakia combined with type 2 diabetes mellitus is associated with a chronic proinflammatory shift in the local microenvironment. The diabetic metabolic profile, characterized by persistent hyperglycemia, endothelial dysfunction, and redox imbalance, contributes to epithelial instability and impaired apoptotic regulation in precancerous lesions of the oral cavity.Histological progression of leukoplakia in this cohort is frequently accompanied by sustained overexpression of tumor necrosis factor alpha (TNF-α), interleukins IL-1β, IL-6, IL-17, IL-23, and vascular endothelial growth factor (VEGF), which modulate signaling axes involved in cell cycle control and matrix degradation. Elevated activity of matrix metalloproteinases MMP-8, MMP-9, and MMP-13 facilitates invasion by altering epithelial–stromal interactions and compromising basement membrane integrity [1,2].Microbial analysis demonstrates consistent colonization by anaerobic periodontopathogens such as Fusobacterium nucleatum, Prevotella spp., and Porphyromonas gingivalis, organisms with documented capacity to induce β-catenin and NF-κB signaling through adhesion and proteolytic activity. In biopsy material from dysplastic oral mucosa, their presence correlates with increased proliferative indices and immune evasion markers [3].The interdependence of cytokine-mediated inflammation, metabolic disturbance, and microbial virulence forms a synergistic framework sustaining epithelial dysplasia and malignant transformation. The present study examines the mechanistic role of inflammatory and growth regulatory mediators in this process, with emphasis on molecular pathways specific to the diabetic leukoplakic oral epithelium [2,4].Chronic low-grade inflammation in type 2 diabetes mellitus (T2DM) plays a key role in promoting epithelial instability in oral potentially malignant disorders. The persistent hyperglycemic state leads to increased expression of proinflammatory cytokines such as TNF-α, IL-1β, and IL-6, which stimulate mitogenic signaling pathways, inhibit apoptotic mechanisms, and modulate the tumor microenvironment [1]. These mediators contribute to molecular dysregulation of the oral epithelium and accelerate the transformation of leukoplakic lesions into squamous cell carcinoma [3,5].Vascular endothelial growth factor (VEGF), together with matrix metalloproteinases MMP-8, MMP-9, and MMP-13, has been found to be significantly elevated in oral tissues of patients with coexisting leukoplakia and T2DM. These factors promote neovascularization, stromal remodeling, and extracellular matrix degradation, facilitating local invasion and epithelial–mesenchymal transition [2]. The imbalance between growth-promoting signals and immune surveillance in diabetic patients may explain the increased frequency and aggressiveness of malignant transformation in oral leukoplakia [1,4].The oral microbiome further amplifies this pathogenic process. An increased abundance of Fusobacterium nucleatum, Prevotella spp., and Porphyromonas gingivalis has been reported in the diabetic oral cavity. These species produce virulence factors capable of activating NF-κB and β-catenin pathways, enhancing proinflammatory cytokine expression and promoting proliferative dysplasia [3]. Their presence in leukoplakic and dysplastic mucosa is associated with increased epithelial proliferation, angiogenesis, and matrix degradation [4].Multiple studies have also demonstrated the association between dysregulated cytokine signaling and increased expression of oncogenic markers such as Ki-67 and p53 in diabetic patients with oral precancerous conditions [5]. These molecular changes reflect a systemic alteration in immune response and tissue regeneration capacity, driven by both metabolic and microbial stimuli.
2. Materials and Methods
- The study was conducted from 2018 to 2020 at the Department of Head and Neck Tumors, N.N. Blokhin National Medical Research Center of Oncology. A total of 150 individuals were enrolled. The main group included 100 patients with histologically verified squamous cell carcinoma of the oral mucosa, arising in the context of clinically and histologically confirmed leukoplakia and comorbid type 2 diabetes mellitus. This group was stratified into two subgroups: patients with newly diagnosed tumors (n = 50) and patients with locoregional recurrences or residual neoplastic growth following prior treatment (n = 50). The control group (n = 50) consisted of diabetes-free volunteers without clinical or histological signs of oral mucosal pathology.Prior to any therapeutic intervention, two mucosal samples per patient were obtained using sterile applicators: one from the tumor surface and one from an anatomically intact site. Samples were placed into anaerobic transport media and processed within one hour. Aerobic and anaerobic culturing was performed on Schaedler agar, blood agar, Endo medium, chocolate agar, and thioglycollate broth. Anaerobic incubation was carried out using AnaeroGen and GasPak systems at 37°C for 72 hours. Colony morphology and purity were evaluated by light microscopy. Identification was performed by MALDI-TOF mass spectrometry (Microflex LT, Bruker Daltonics). Antimicrobial susceptibility was tested using automated analyzers MicroScan WalkAway 40/96 and Vitek-2. Interpretation of minimum inhibitory concentrations followed EUCAST guidelines (v8.1).Histological specimens obtained during surgical excision were examined for epithelial dysplasia, basal membrane disruption, inflammatory infiltration, vascular density, and mitotic index. Paraffin-embedded sections were stained with hematoxylin–eosin and processed for immunohistochemistry using antibodies against TNF-α, IL-1β, IL-6, IL-17, IL-23, VEGF, and MMP-9. Detection was achieved using the Dako EnVision system. Ki-67, β-catenin, and p53 were assessed semi-quantitatively in basal and suprabasal epithelial layers.Quantification of cytokine gene expression was carried out by qPCR. RNA was isolated from fresh tumor and control mucosa using the RNeasy Mini Kit (Qiagen). cDNA synthesis was performed with High-Capacity Reverse Transcription Kits (Thermo Fisher Scientific). Amplification was conducted on a StepOnePlus real-time PCR system using TaqMan assays. Normalization was performed against GAPDH. Fold changes were calculated using the 2^-ΔΔCt method.Statistical analysis employed SPSS v.25.0. Intergroup comparisons were conducted using the Mann–Whitney U test or Kruskal–Wallis test. Categorical variables were analyzed via Pearson’s χ² test or Fisher’s exact test. Correlation between microbial species prevalence, cytokine expression levels, and clinical-pathological parameters was assessed using Spearman’s rank correlation coefficient. Statistical significance was accepted at p < 0.05.
3. Results and Discussion
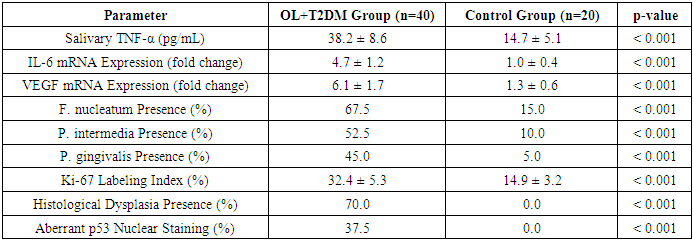
- The study encompassed 60 participants, divided into two groups: 40 patients diagnosed with oral leukoplakia (OL) concomitant with type 2 diabetes mellitus (T2DM), and 20 age- and sex-matched healthy controls without diabetes or oral mucosal lesions. The mean age in the OL+T2DM group was 61.4 ± 7.2 years, while in the control group it was 59.3 ± 6.5 years.Quantitative real-time PCR (qRT-PCR) analysis of oral mucosal biopsy specimens revealed significantly elevated mRNA expression levels of pro-inflammatory cytokines and growth factors in the OL+T2DM group compared to controls. Specifically, TNF-α expression was increased by 5.4-fold, IL-6 by 4.7-fold, and VEGF by 6.1-fold (p < 0.001 for all).Enzyme-linked immunosorbent assay (ELISA) of salivary samples corroborated these findings, showing higher protein concentrations of the aforementioned cytokines in the OL+T2DM group. Mean salivary TNF-α levels were 38.2 ± 8.6 pg/mL in the OL+T2DM group versus 14.7 ± 5.1 pg/mL in controls (p < 0.001).Microbiological analysis demonstrated a higher prevalence of pathogenic bacteria in the OL+T2DM group. Fusobacterium nucleatum was detected in 67.5% of patients, Prevotella intermedia in 52.5%, and Porphyromonas gingivalis in 45%, compared to 15%, 10%, and 5% respectively in controls (p < 0.001 for all).Histopathological examination indicated that 70% of OL+T2DM patients exhibited mild to moderate epithelial dysplasia. Immunohistochemical analysis showed increased Ki-67 labeling index (32.4 ± 5.3%) and aberrant p53 nuclear staining in 37.5% of cases, suggesting enhanced cellular proliferation and potential genomic instability.These findings suggest a synergistic interplay between chronic inflammation, metabolic dysregulation, and microbial colonization in the oral mucosa of patients with OL and T2DM, potentially facilitating carcinogenic transformation.
|
4. Conclusions
- The present investigation highlights the multifactorial nature of oral carcinogenesis in patients with coexisting leukoplakia and type 2 diabetes mellitus. The data demonstrate that persistent hyperglycemia, microbial dysbiosis, and chronic inflammation converge to promote epithelial proliferation, angiogenesis, and dysplastic transformation of the oral mucosa. Significant overexpression of TNF-α, IL-6, and VEGF, alongside elevated Ki-67 and p53 activity, reflects a high-risk molecular profile. The increased colonization by Fusobacterium nucleatum and P. gingivalis, coupled with histologically confirmed dysplasia in 70% of diabetic leukoplakia cases, underscores the pathogenetic synergy between systemic metabolic dysfunction and local mucosal immunoinflammatory response. These findings support the integration of cytokine profiling and microbial diagnostics into routine clinical assessment of high-risk patients and provide a rationale for targeted prevention strategies. Future studies should focus on longitudinal surveillance and therapeutic modulation of cytokine and microbiota-mediated signaling pathways.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML