-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(6): 2074-2078
doi:10.5923/j.ajmms.20251506.99
Received: May 25, 2025; Accepted: Jun. 22, 2025; Published: Jun. 26, 2025

Methods of Diagnosing Lactase Enzyme Deficiency Disease in Children at an Early Age Using Modern Examination Methods
Khidirova Sojida Husniddinovna
PhD Student, Department of Normal and Pathological Physiology, Samarkand State Medical University, Samarkand, Uzbekistan
Correspondence to: Khidirova Sojida Husniddinovna, PhD Student, Department of Normal and Pathological Physiology, Samarkand State Medical University, Samarkand, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Relevance of the Topic. Three-quarters of the world’s population cannot digest lactose, and consumption of dairy products can cause various gastrointestinal problems. Genetic testing of the LCT (-13910) C>T rs4988235 polymorphism is the most reliable and effective method for diagnosing lactose intolerance. By identifying the C>T genetic polymorphism (-13910) of the LCT gene, the activity of the enzyme can be evaluated using PCR (polymerase chain reaction) technology for DNA analysis. Materials and Methods. For this study, 90 children aged from under 1 year to school age (mean age 1 ± 7) diagnosed and treated for lactase deficiency at the Pediatric Gastroenterology Department of the Multidisciplinary Children’s Hospital in Samarkand region were selected. Alleles and genotypes of the LCT gene C>T -13910 rs4988235 polymorphism were determined using blood samples taken from the venous blood vessels and analyzed with the “NПФ LITEX” reagents from Russia. Genetic analysis and data evaluation were conducted following GRIPS guidelines, which help reduce risk bias and improve data quality. Statistical analysis of the results was performed using Stat Soft Statistica 10.0 software (USA). The deviation of genotype distributions from Hardy-Weinberg equilibrium was assessed using the “Gene Pop” (Genetics of Population) computer program. Results. According to the applied testing methods, groups were formed based on the frequency distribution of alleles and genotypes of the C>T –13910 polymorphism in children of early age in Uzbekistan, considering disease severity, progression, and etiology. The main group consisted of 87.78% C/C genotype, 10% C/T genotype, and 2.2% T/T genotype. Conclusion. Studying the distribution of alleles and genotypes in the population revealed that the C allele predominantly contributes to severe lactose malabsorption, while the T allele plays a role in maintaining the ability to digest lactose later in life.
Keywords: Polymorphisms, Lactose intolerance, LCT, PCR diagnostics
Cite this paper: Khidirova Sojida Husniddinovna, Methods of Diagnosing Lactase Enzyme Deficiency Disease in Children at an Early Age Using Modern Examination Methods, American Journal of Medicine and Medical Sciences, Vol. 15 No. 6, 2025, pp. 2074-2078. doi: 10.5923/j.ajmms.20251506.99.
Article Outline
1. Introduction
- Lactase deficiency is a pathology of the small intestine, characterized by the absence or insufficient activity of the lactase enzyme, leading to the development of malabsorption syndrome. Lactase deficiency was first described by Hippocrates in the 400s BC, but its clinical significance has only been deeply studied in the past 50 years. Lactase deficiency is one of the most widespread metabolic disorders, affecting nearly 4 billion people worldwide. The amount of lactose that causes clinical symptoms varies according to the degree of lactase deficiency. The major significance of the problem is that milk and dairy products are predominant in the daily diet of newborns and young children. Lactase deficiency can be primary or secondary. Primary lactase deficiency occurs due to a genetic (congenital) absence of lactase enzyme. Secondary enzyme deficiency arises as a result of damage to the small intestine's mucosa, often following endocrine disorders, extensive intestinal resection, parasitic diseases, and various infectious intestinal diseases, which lead to insufficient enzyme production from intestinal enterocytes. The frequency of lactose intolerance (LI) varies in the population from 5% to 100%, being least common among residents of England [1], more common among Basques—over 90% [2], and in Russia—around 15% of the adult population [3]. Among people aged 2 to 10 years, LI is observed in 6-15% of Americans and residents of Northern Europe, 18-47% of residents of Mexico, 25-60% of South Africans, 10-25% of Chinese and Japanese, and 30% or more among residents of Peru [4,5]. In Siberia and the Far East, up to 90% of children under one year old show signs of lactose intolerance [6-8]. Clinical manifestations of LI are associated with the following factors: insufficient breakdown of the disaccharide in the small intestine, impaired absorption of monosaccharides, and disturbances in the intestinal microbiota.Classification of Lactose Intolerance (LI)I. By degree of severity: partial (hypolactasia) and complete (alactasia)II. By originPrimary – reduction in lactase activity with intact enterocytes. The following variants are noted:ο Transitory LI in premature and underdeveloped infants at birth, associated with the temporary immaturity of enterocytes and the absence or insufficient production of lactase.ο Congenital (familial or genetically determined), a neonatal urgent condition, inherited in an autosomal recessive manner, occurring with a frequency of 1 in 60,000, and caused by mutations in the coding region of the lactase gene [9].ο Adult-type LI (constitutional). The mechanisms of adult-type LI and congenital LI are the same: impaired activation of the enzyme and its expression on the membrane, leading to the accumulation of its precursor in the Golgi complex [10].Secondary LI – a reduction in lactase activity associated with damage to enterocytes, accounting for about 50-80% of all its manifestations. The high prevalence of secondary lactose intolerance (LI) is explained by the disruption of enzymatic activity in enterocytes due to the influence of infectious factors. This affects the condition of the brush border layer of the mucosa, the migration rate of enterocytes, the degree of differentiation and maturation of cells, and the state of the glycocalyx. The main causes of secondary LI development are: Infectious processes (intestinal infections of viral and bacterial origin), Giardiasis, helminth infestations, Immune processes (cow's milk protein intolerance), Inflammatory processes in the intestine, Disorders of local immunity (deficiency of secretory IgA), Chronic inflammatory diseases of the colon (ulcerative colitis, Crohn's disease), Atrophic changes, for example, after a prolonged period of total parenteral nutrition, or in celiac disease.The functional state of the small intestine's mucosal layer and the amount of lactase enzyme are influenced by the following factors: [34]1. Gestational age - Most common in premature and underdeveloped newborns. 2. Genetic factors - Polymorphism variants of the lactase synthesis responsible gene (LCT).3. Hormonal factors.4. Vegetative nervous system.5. Growth factors related to protein nature.Physiology of Lactase Lactase is first detected during the 10th–12th week of gestation, and by the 24th week, its activity begins to increase, reaching its peak at the time of birth [11]. In the final weeks of gestation, lactase activity continues to rise, surpassing adult levels. The reduction in enzyme activity begins at the end of the first year of life, and by the age of 2, lactase activity becomes inversely proportional to age. During the preschool years, its level remains stable, and after 5 years, the decline becomes more pronounced [12]. Between the ages of 10 and 18, the enzyme level reaches a point that will persist throughout the individual's life [13]. Lactase distribution along the "villus-crypt" axis is uneven. Cells from the crypt zone, which is the area of enterocyte proliferation, move towards the apex of the villi, during which differentiation occurs. They reach full development at the villus tip. Lactase of the brush border, compared to other disaccharidases, is located closer to the villus tip, especially in the duodenum [14]. This explains the more frequent occurrence of secondary LI when the mucosa is damaged by any etiology, compared to a deficiency of other enzymes. Maximum lactase activity is observed in the distal parts of the jejunum [11]. Lactase is the only enzyme in the human body that breaks down lactose. It also has other enzymatic activities, such as phlorizdin hydrolase, glycosylceramidase, and β-galactosidase [15]. This allows lactase to participate in the breakdown of glycolipids. It has been shown that lactase activity is primarily associated with the lactase-phloridzin hydrolase enzyme, which is the main glycoprotein on the microvilli membrane. This protein has two enzymatic activities: lactase (β-D-galactosidase), responsible for lactose breakdown, and phloridzin hydrolase (glycosyl-N-acetylsphingosine glucohydrolase), which breaks down phloridzin, a compound believed to regulate monosaccharide absorption. The enzyme passes through the membrane via a hydrophobic sequence at the COOH-end and functions in the glycocalyx [10]. Lactase breaks down the primary carbohydrate in human milk, which makes up 85% of the total carbohydrate content. The remaining 15% consists of other oligosaccharides. Lactose is a disaccharide composed of two monosaccharides—glucose and galactose, which it is broken down into in the small intestine by lactase. A characteristic feature of human milk lactose is its β configuration, unlike the α-lactose found in cow's milk, which is formed when β-lactose is converted in the presence of significant amounts of phosphate ions found in cow's milk. Lactose helps with calcium absorption, has bifidogenic properties, and lowers the pH in the colon. β-lactose is digested more slowly than α-lactose. As a result, it reaches the small and partially the large intestine in an undigested form, where it serves as a substrate for intestinal bifidobacteria and lactobacilli. These microorganisms ferment lactose into short-chain fatty acids, lactic acid, carbon dioxide, methane, hydrogen, and water [16]. It should be noted that the presence of undigested lactose in the large intestine occurs even in full-term newborns, despite the fact that lactase levels in the small intestine are at their highest. This is confirmed by increased hydrogen content in the exhaled air of full-term newborns up to 3 months of age, reaching levels typical of adults with lactose intolerance, despite the absence of clinical symptoms [17].The gene encoding enzyme synthesis, LCT, is located on chromosome 2q21-22. Single nucleotide polymorphisms (SNPs) in the intron region of the MСM6 gene, approximately several kilobases away from the transcription start site of the LCT gene, influence phenotypic outcomes. The nucleotide positions (13910 (C/T-13910) substitution of cytosine (C) to thymine (T) and adenine (A) to guanine (G) (G/A-22018)) determine phenotypes.
2. Purpose of the Study
- The aim of the study is to investigate the role of lactase enzyme in metabolic processes and the development of unpleasant symptoms in diagnosed cases. It also aims to differentiate from other diseases caused by enzyme activity disorders and introduce modern methods for correct diagnosis in practice.
3. Materials and Methods
- The study was conducted on 90 children diagnosed with lactase enzyme deficiency at the Children's Gastroenterology Department of the Samarkand Regional Multidisciplinary Medical Center. Children under school age were separated for the study. Based on the goals and objectives of the research, blood samples were taken from patients of various age groups (from neonatal period to 7 years of age). The allele and genotype distribution frequencies of the LCT gene polymorphisms were determined. Blood samples were collected from the venous system of the patients, placed in special test tubes, and DNA was extracted using PCR diagnostic methods. Special reagents were used for testing. For the analysis of LCT C-13910T rs4988235, reagents from НПФ 'LITEX' – Russia were used. The DNA fragments spanning the C/T-13910 and G/A-22018 variants, respectively, were amplified using one biotinylated (5′-CCTCGTTAATACCCACTGACCTA-3′ for C/T-13910 variant and 5′-TGCTCAGGACATGCTGATCAA-3′ for G/A-22018 variant) and one unbiotinylated (5′-GTCACTTTGATATGATGAGAGCA-3′ for C/T-13910 variant and 5′-CTACCCTATCAGTAAAGGCCTA-3′ for G/A-22018 variant) primer. The families/children gave their informed consent.
4. Results and Discussion
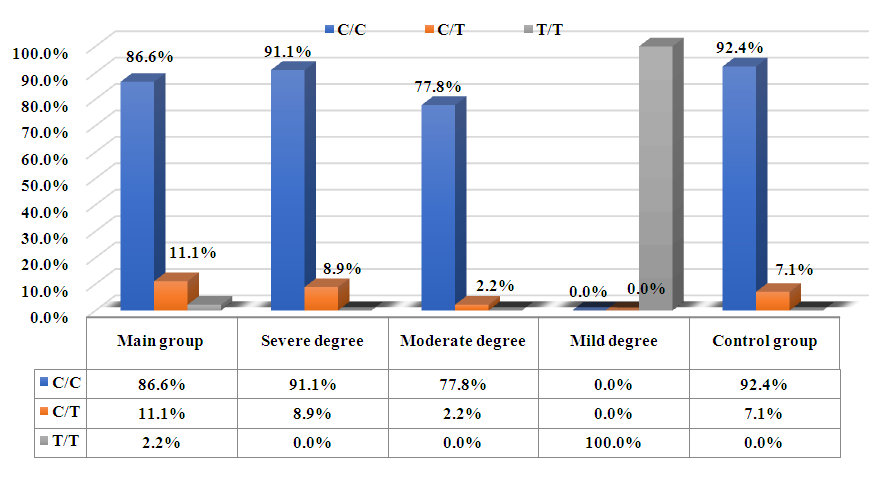
- Among the 90 patients diagnosed with the disease and 85 healthy volunteers, the main and control groups were formed. Statistical indicators were determined based on the distribution of alleles and genotypes in both groups. The results of the study related to the C>T-13910 polymorphism are shown in the following. The research was conducted in the molecular-genetic laboratory department of the Republican Specialized Hematology Scientific-Practical Medical Center."
|
 | Figure 1 |
5. Conclusions
- Based on the above table, it can be concluded that the three genotypes of the C>T 13910 polymorphism directly affect the development of the disease. The C/C genotype was found in 91.1% of severe cases, the C/T genotype was most commonly found in 77.8% of moderate cases, and the T/T genotype was observed exclusively in 100% of mild cases. The C allele and C/C genotype are more prevalent in individuals with severe and moderate forms of lactase deficiency. The presence of the T/T genotype was observed only in the mild cases, suggesting that the T allele might be associated with milder forms of the condition. The Control Group displayed a typical distribution of genotypes and alleles found in a healthy population, where the C allele was dominant. Overall, the distribution of genotypes indicates that the C/C genotype is strongly associated with more severe cases of lactase deficiency, while T/T is seen exclusively in mild cases.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML