-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(6): 2037-2042
doi:10.5923/j.ajmms.20251506.91
Received: May 28, 2025; Accepted: Jun. 19, 2025; Published: Jun. 23, 2025

Clinical And Laboratory Assessment of the Impact of Subclinical Hypothyroidism on the Severity of Hypertensive Disorders in Pregnant Women (Based on Data from Andijan Region)
Usmanova Mokhinur Dilshod qizi1, Nasirova Feruza Jumabaevna2, Yakubova Oltinoy Abduganievna3
1Independent Researcher, Department of Obstetrics and Gynecology No.1, Andijan State Medical Institute, Andijan, Uzbekistan
2Doctor of Medical Sciences, Associate Professor, Department of Obstetrics and Gynecology No.1, Andijan State Medical Institute, Andijan, Uzbekistan
3Doctor of Medical Sciences, Associate Professor of Advanced Training and Retraining Faculty, Department of Obstetrics and Gynecology, Traumatology-Orthopedics, and Neurosurgery, Andijan State Medical Institute, Andijan, Uzbekistan
Correspondence to: Usmanova Mokhinur Dilshod qizi, Independent Researcher, Department of Obstetrics and Gynecology No.1, Andijan State Medical Institute, Andijan, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Subclinical hypothyroidism may represent a significant risk factor for hypertensive disorders during pregnancy. In the Andijan region, which is endemic for iodine deficiency, subclinical hypothyroidism is widespread; however, its association with hypertensive disorders remains insufficiently explored. This study aimed to evaluate the impact of subclinical hypothyroidism on the severity of hypertensive disorders in pregnant women. A total of 88 women with hypertensive disorders were examined and stratified by thyroid status: subclinical hypothyroidism (n = 46) and euthyroidism (n = 42). Anamnestic, clinical, laboratory, ultrasound data, and somatic symptoms were analyzed. Statistical processing was performed using the R program. Significant differences were identified between the groups in terms of age, frequency of severe preeclampsia, adverse obstetric outcomes, and levels of TSH, thyroglobulin, and anti-TPO antibodies. Women with subclinical hypothyroidism more frequently reported somatic symptoms, thyroid enlargement, and pronounced edema syndrome. The findings confirm the impact of subclinical hypothyroidism on the clinical course of hypertensive disorders and underscore the importance of thyroid screening in pregnant women living in iodine-deficient regions.
Keywords: Pregnancy, Subclinical hypothyroidism, Hypertensive disorders, Thyroid hormones, Andijan region
Cite this paper: Usmanova Mokhinur Dilshod qizi, Nasirova Feruza Jumabaevna, Yakubova Oltinoy Abduganievna, Clinical And Laboratory Assessment of the Impact of Subclinical Hypothyroidism on the Severity of Hypertensive Disorders in Pregnant Women (Based on Data from Andijan Region), American Journal of Medicine and Medical Sciences, Vol. 15 No. 6, 2025, pp. 2037-2042. doi: 10.5923/j.ajmms.20251506.91.
1. Introduction
- Hypertensive disorders (HD) during pregnancy remain one of the leading causes of maternal and perinatal morbidity. According to WHO, they occur in an average of 10% of pregnant women, and in low- and middle-income countries, this figure may exceed 17% [1,2,3]. During pregnancy, significant hormonal changes take place, including alterations in thyroid function. Even subclinical forms of thyroid dysfunction, such as subclinical hypothyroidism (SCH), can disrupt hormonal homeostasis and contribute to the development of complications, including preeclampsia [4,5]. SCH is characterized by elevated TSH levels with normal free T4 levels and is considered one of the factors in vascular dysfunction.In Uzbekistan, especially in iodine-deficient regions, the prevalence of SCH among pregnant women is estimated to be between 7–15%, and in the Andijan region around 12% [6,7]. Despite measures to prevent iodine deficiency, the coverage of iodine prophylaxis remains insufficient. Andijan region is an endemic area for iodine deficiency, which accounts for the high prevalence of SCH among women of reproductive age. However, the association between SCH and hypertensive complications of pregnancy in this region remains poorly studied.Considering the multifactorial nature of HD risk including endocrine, genetic, environmental, and social aspects investigating the role of SCH in the development of hypertensive conditions becomes especially relevant. This is essential for improving diagnostics, prevention, and pregnancy management in the context of regional iodine deficiency.The aim of the study was to assess the impact of SCH on the occurrence and course of hypertensive disorders in pregnant women in the Andijan region.
2. Materials and Methods
- From November 2022 to April 2023, a case–control study was conducted at the Andijan branch of the Republican Specialized Scientific and Practical Center for Maternal and Child Health among pregnant women with hypertensive disorders (HD).Inclusion and exclusion criteria: Included were women aged 18–40 years, with singleton pregnancies, in whom HD was diagnosed after the 20th week. Cases of chronic hypertension were excluded (N=88).Data collection: Data were collected via an online questionnaire using the KoBoToolbox platform, which included sociodemographic, clinical, and laboratory parameters. As part of a comprehensive examination, patients underwent fetal and thyroid Doppler studies, clinical examination with assessment of somatic status, and laboratory investigations. On the second day of hospitalization, fasting venous blood samples were collected for the determination of TSH, triiodothyronine (T3), thyroxine (T4), thyroglobulin, anti-thyroid peroxidase antibodies (anti-TPO), and anti-thyroglobulin antibodies (anti-TG).Diagnosis of SCH and HD was made according to the national guidelines of the Ministry of Health of Uzbekistan [7,8]. SCH was diagnosed with TSH > 4.2 μIU/mL and normal T4 (10.3–24.5 pmol/L) combined with elevated anti-TPO (>18 IU/mL). HD was defined as BP ≥140/90 mmHg without proteinuria; preeclampsia with proteinuria ≥0.3 g/L; severe preeclampsia with BP ≥160/110 mmHg and signs of organ dysfunction.Ethical approval: The study was approved by the Ethics Committee of the Ministry of Health of the Republic of Uzbekistan (No. 1684/6-1 dated 27.09.2022).Statistical analysis: Data were processed using R software (v.4.2.1) with tidyverse, rio, and here packages. Statistical differences were assessed using the Chi-squared test, Fisher’s exact test, and Wilcoxon rank-sum test. The significance level was set at p<0.05.
3. Results
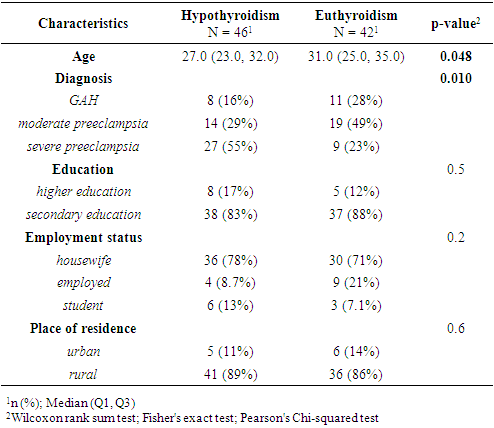
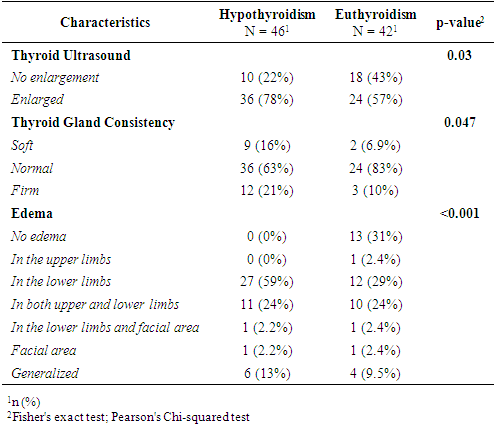
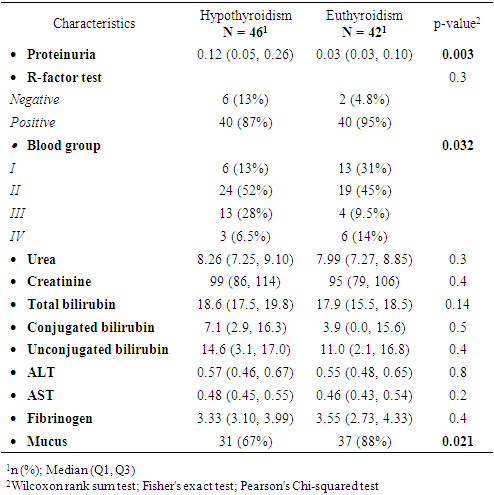
- Based on thyroid profiles and endocrinologist consultations, participants with HD were divided into two groups: those with SCH (n = 46) and those with euthyroidism (n = 42). Comparative analysis revealed statistically significant differences in age and types of HD. Women with SCH were younger (27.0 [23.0–32.0] years) compared to euthyroid patients (31.0 [25.0–35.0] years; p = 0.048), which may indicate an earlier manifestation of thyroid dysfunction. The structure of hypertensive conditions also differed (p = 0.01): in the euthyroid group, HD and mild preeclampsia were more frequently diagnosed (28% and 49%, respectively), while in the SCH group, severe preeclampsia predominated (55% vs. 23%). This may indicate more pronounced vascular disturbances in the presence of thyroid dysfunction. Sociodemographic parameters (education, employment, place of residence) showed no significant differences (p > 0.05), indicating no apparent influence on the development of SCH in this sample (Table 1).
|
|
|
|
|
|
4. Discussion
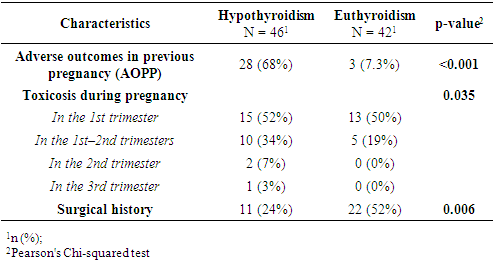
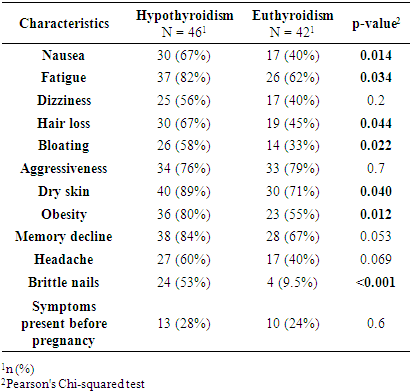
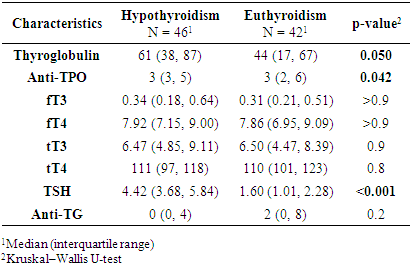
- This study revealed statistically significant differences between pregnant women with subclinical hypothyroidism (SCH) and those with euthyroidism in terms of age, severity of hypertensive disorders, frequency of clinical symptoms, and several laboratory and instrumental parameters. It was established that women with SCH were younger and significantly more likely to experience severe forms of preeclampsia. These findings are consistent with previous studies by Casey et al. (2005) and Wilson et al. (2012) [9,10], where SCH was considered an independent risk factor for complicated preeclampsia. Socio-demographic characteristics such as education, place of residence, and employment did not significantly influence the development of thyroid dysfunction, as also reflected in regional data from Central Asia [11].Women with SCH were more likely to have had adverse outcomes in previous pregnancies, consistent with findings from Gietka-Czernel et al. (2021) [12]. Prolonged symptoms of toxicosis and a lower frequency of surgical interventions in anamnesis also require further investigation of underlying pathophysiological mechanisms. Somatic symptoms were significantly more common in the SCH group, including nausea, fatigue, hair loss, dry skin, obesity, and brittle nails all classical manifestations of hypothyroid states, as confirmed by clinical observations (Nazarpour et al., 2019) [13]. Meanwhile, nonspecific symptoms (dizziness, memory issues, emotional instability) did not differ between groups, indicating limited diagnostic value in assessing thyroid status.The hormonal profile of SCH patients showed elevated levels of TSH, thyroglobulin, and thyroid autoantibodies, while free and total T3 and T4 levels remained stable suggesting a compensated phase of thyroid dysfunction [14]. Ultrasound findings more frequently showed enlarged thyroid volume and altered consistency in SCH patients, possibly indicating structural remodeling of autoimmune origin. Edema syndrome was significantly more prevalent in SCH patients, including both localized and generalized forms, reflecting fluid retention and impaired water-electrolyte balance typical of hypothyroidism, as described in the MSD Manual [15]. Laboratory analysis also revealed higher levels of proteinuria in SCH patients, suggesting endothelial dysfunction and impaired glomerular filtration, which has been described in cases of membranous nephropathy [16]. Differences in blood group distribution and mucus presence in urine also emerged, though they require further investigation and may reflect individual metabolic characteristics associated with SCH.
5. Conclusions
- In conclusion, SCH in pregnant women is associated with more severe hypertensive complications, pronounced clinical manifestations, and notable laboratory deviations. These results support the inclusion of thyroid function screening and monitoring in standard pregnancy management protocols, especially in iodine-deficient regions.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML