Nurbaev F. E., Kadirova M. N.
Bukhara State Medical Institute, Bukhara, Uzbekistan
Correspondence to: Nurbaev F. E., Bukhara State Medical Institute, Bukhara, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
This article studied 183 women of reproductive age who were treated as inpatients in the hematology department of the Bukhara Regional Multidisciplinary Medical Center, divided into two groups. The results obtained confirm that the appointment of antianemic therapy after antibacterial treatment in women of childbearing age with iron deficiency is highly effective. In contrast, positive changes were observed only in the group receiving iron preparations for anemia, but the differences were not highly reliable. When comparing the two groups, it was noted that the control group was more reliable in the active group than in the control group. This confirms that women of childbearing age with anemia diagnosed with Helicobacter pylori should first undergo eradication therapy and then recommend antianemic treatment. In this case, although morphological changes in erythrocytes are stable for a long time, some results showed that they are reliable.
Keywords:
Helicobacter pylori, Iron deficiency anemia, Women of reproductive age, Antiarrhythmic drugs, Proton pump inhibitor eradication therapy, Amoxicillin, Clarithromycin, Metronidazole, Bismuth preparations and ferrokinetic indicators
Cite this paper: Nurbaev F. E., Kadirova M. N., Treatment of Helicobacter Pylori Associated Iron Deficiency Anemia in Women of Reproductive Age, American Journal of Medicine and Medical Sciences, Vol. 15 No. 6, 2025, pp. 2032-2036. doi: 10.5923/j.ajmms.20251506.90.
1. Introduction
According to the World Health Organization (WHO), there are 1.62 billion patients with anemia in the world, which is 24.8% of the population [1]. Among them, about 50% are people with iron deficiency anemia [2,3]. Iron deficiency anemia is a disease characterized by a decrease in the level of the trace element in the blood serum, tissue stores, bone marrow and other organs, which leads to the development of hypochromia and trophic disorders [4,5].This type of anemia is the most common and most common type of anemia in women of reproductive age [6]. According to the above-mentioned international organization, anemia is observed in one in three women of reproductive age and in one in two pregnant women, and is an important cause of chronic fatigue and poor health. It is the third leading cause of temporary disability among women aged 15-44 [7].The main causes of iron deficiency anemia in women of childbearing age are heavy menstrual bleeding, pregnancy, frequent and short-term childbirth, uterine fibroids, adenomyosis, endometrium, and lactation. In cases of normal menstruation, 30-40 ml of blood is lost. This corresponds to approximately 15-20 mg of iron. If 40-60 ml of blood is lost during this process, it is considered severe. If it is more, iron deficiency develops. In sick women with abnormal uterine bleeding, blood loss can reach 200 ml. This corresponds to 100 mg of iron lost with blood. In this process, iron loss gradually exceeds the intake of iron into the body, and iron deficiency anemia develops in a woman [8]. Anemia, especially observed in pregnant women, is of particular medical and social importance. According to the World Health Organization, 24-30% of pregnant women in developed countries and 50% of pregnant women in developing countries are diagnosed with this disease [9].Fifteen percent of patients have unexplained or refractory iron deficiency anemia. [11] This refractory anemia has been shown to be caused by H. pylori in a number of cases.It is known that this bacterium is one of the most common infections in the world, affecting 50% of the population in developed countries and 90% of the population in developing countries [12,13,14,15]. According to studies, H. pylori is most common in Africa, and to a lesser extent in Latin American countries [16,17,18]. In contrast, in Western Europe and Australia, H. pylori infection is less common and amounts to 30-40% [19,20]. According to data, this infection is recorded in 50-80% of the population of the Russian Federation, and its prevalence varies somewhat in different regions. Some researchers have found that the prevalence of H. pylori varies somewhat among the population of different ages [21,22].In our country, anemia is widespread among women of childbearing age and pregnant women, and a number of its aspects have been studied. However, so far, there is no information in the literature we have reviewed about iron deficiency anemia associated with H. pylori in women of reproductive age.Purpose: Study on the treatment of iron deficiency anemia associated with Helicobacter pylori in women of reproductive age.
2. Materials and Styles
183 women of reproductive age who were treated as inpatients in the hematology department of the Bukhara Regional Multidisciplinary Medical Center were divided into two groups. Comparative indicators of ferrokinetic and inflammatory cytokines were analyzed before and after treatments in patients in the main and control groups.
3. Results
As we described in our scientific work, the main group of patients in our observation group, in which Helicobacter pylori was detected, were divided into two groups based on the treatments performed.The first main group of them consisted of 100 women with an average age of 36.2±3.5 years. This group of patients was initially prescribed eradication therapy against Helicobacter pylori (proton pump inhibitor, amoxicillin, clarithromycin, metronidazole, bismuth preparations) in individual doses. After the bacterial test was negative after complex treatment against Helicobacter pylori, iron III sucrose complex was recommended as an antianemic drug.The second group included 83 women with a mean age of 37.4±2.6 years. They were prescribed only antianemic treatment (iron III sucrose complex in an individual dose for each patient based on serum iron levels). The results were compared and are presented in Table 1 below.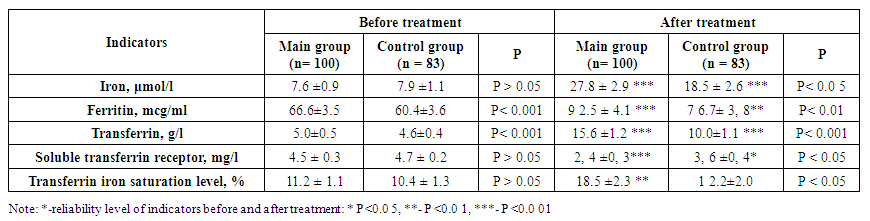 | Table 1. Comparison of pre- and post-treatment ferrokinetic and inflammatory cytokines in primary and control patients |
As shown in the table, the serum iron content in the main and control groups before treatment was 7.6±0.9 and 7.9±1.1 μmol/l, respectively. When comparing them, no significant difference was detected (P>0.05). After the staged complex treatment in the main group, a 3.65-fold increase was noted from 7.6±0.9 μmol/l to 27.8±2.9 μmol/l, a highly significant difference (P<0.001). In the control group, the serum iron content increased from 7.9±1.1 μmol/l to 18.5±2.6 μmol/l, a highly significant difference was observed (P<0.001). When comparing serum iron values after treatment between the groups, a significant difference was observed (P<0.05). Ferritin levels in the main group before treatment were 66.6±3.5 μg/ml and after treatment were 92.5±4.1 μg/ml, with a significant difference (P<0.001). In the control group, its level increased significantly (P<0.01) from 60.4±3.6 to 76.7±3.8 μg/ml after anti-anemic treatment. When comparing ferritin levels before and after treatment in the main and control groups, a significant difference was noted (P<0.01).The serum transferrin level before treatment was 5.0±0.5 g/l in the main group and 4.6±0.4 g/l in the control group, with no significant difference (P>0.05) between them. After treatment, the transferrin level increased by 15.6±1.2 g/l in the main group with a high significance (P<0.001), and in the control group with a high significance (P<0.001). When comparing the transferrin levels after treatment between the groups, a significant difference (P<0.001) was observed.Soluble transferrin receptors decreased 1.87 times from 4.5±0.3 mg/l to 2.4±0.3 mg/l in patients who received eradication therapy and then antianemic therapy after treatment. When comparing them, a highly significant difference (P<0.001) was noted. In the second group, that is, in patients who received only antianemic therapy, its level before and after treatment was 4.7±0.2 and 3.6±0.4 mg/l, respectively, with a significant difference (P<0.05). When comparing soluble transferrin indicators after treatment in both groups, a significant difference was noted (P<0.05). The level of transferrin iron saturation in the main group was 11.2±1.1% before treatment and 18.5±2.3% after treatment, with a significant difference (P<0.01) between them. In the control group, the levels before and after treatment were 10.4±1.3% and 12.2±2.0%, respectively. However, there was no significant difference (P>0.05) between them. When comparing the levels of transferrin iron saturation after treatment in patients who received and did not receive eradication therapy, a significant difference (P<0.05) was observed.In addition, inflammatory cytokines and Helicobacter pylori markers were also evaluated in both groups of patients after treatment. The results are presented in Figure 1 below.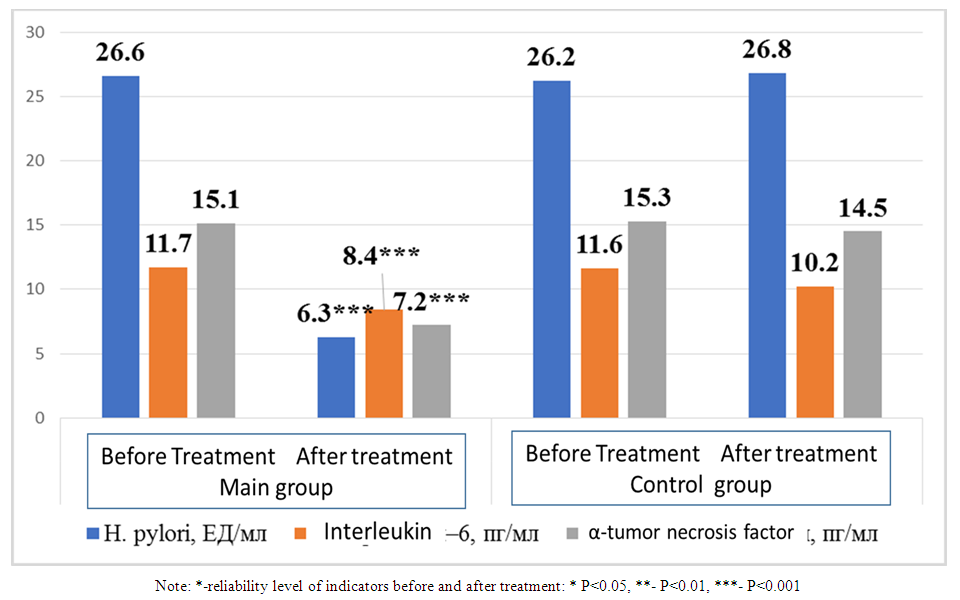 | Figure 1. Comparative analysis of inflammatory cytokines and Helicobacter pylori markers after treatment in patients under observation |
Antibodies to H. pylori before treatment were 26.6±2.1 and 26.2±2.2 U/ml in the main and control groups, respectively (P>0.05). After complex antibacterial treatment, its indicators in the main group decreased to 6.3±0.4 U/ml, and a highly significant difference (P<0.001) was detected. In the group that did not undergo anti-H. pylori treatment, its indicator was 26.8±2.0, and a reliable difference (P>0.05) was not detected. When comparing the H. pylori indicators after treatment in both groups, a highly significant difference (P<0.001) was detected in the first group compared to the second.Serum interleukin-6 levels before treatment were 11.7±0.3 and 11.6±0.2 pg/ml in both groups, respectively, and there was no significant difference (P>0.05). In the main group, its level decreased to 8.4±0.2 pg/ml after treatment, and a highly significant difference was detected (P<0.001). In the control group, its level decreased to 10.2±0.2 pg/ml after treatment, but the differences were not significant. When comparing interleukin-6 levels after treatment in both groups, a highly significant difference (P<0.001) was detected.The levels of α-tumor necrosis factor in the main and control groups before treatment were 15.0±0.3 pg/ml and 15.3±0.2 pg/ml, respectively (P>0.05). After the treatment, its level in the main group decreased significantly (P<0.001) by 7.2±0.2 pg/ml, and in the control group it was 14.5±1.2 pg/ml (P>0.05). When comparing them, a significant difference was found in the first group compared to the second (P<0.001).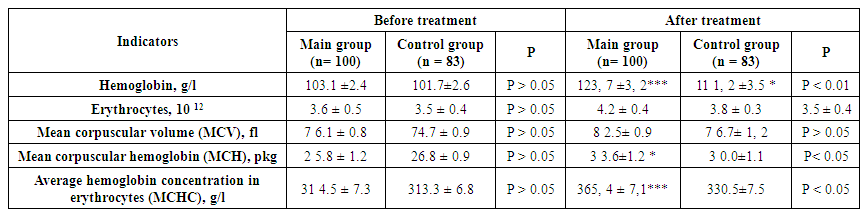 | Table 2. Comparative indicators of morphological indicators of erythrocytes before and after the procedures performed in patients of the main and control groups |
As shown in the table, the hemoglobin level in the main group before treatment was 103.1±2.4 g/l, in the control group - 101.7±2.6. When comparing them, no significant difference was detected (P>0.05). In the main group, after the complex treatment of Helicobacter pylori and anti-anemic treatment, its level increased to 123.7±3.2 g/l, which is 1.2 times, and the difference was highly significant (P<0.001) compared to the pre-treatment indicators. In the control group, which only underwent anti-anemic treatment, it increased to 110.2±2.8 g/l, which is 1.1 times (P<0.05). When comparing the post-treatment indicators in both groups, a significant difference was observed in those who underwent eradication treatments (P<0.01).3.6±0.5*10 12 to 4.2±0.4*10 12 after the complex treatment in the main group, with no significant difference (P>0.05) when compared. In the control group, it was 3.5±0.4*10 12 before the treatment and 3.8±0.3*10 12 after (P>0.05). In both groups, there was no significant difference (P>0.05) when comparing the erythrocyte indices after the treatment.The average volume of erythrocytes in both groups before treatment was 76.1±0.8 and 74.7±0.9 fl, respectively (P>0.05). After the treatments, it was 82.5±0.9 in the first group and 76.7±1.2 fl in the second group. When comparing them, no significant (P>0.05) difference was detected.The average hemoglobin content in erythrocytes in the main group receiving eradication therapy against Helicobacter pylori increased from 25.8±1.2 pkg to 33.6±1.2 pkg after treatment, with a significant difference (P<0.05). In the control group, it was 26.8±0.9 pkg before treatment and 30.0±1.1 pkg after treatment, with no significant difference (P>0.05). When comparing the average hemoglobin content in erythrocytes after treatment in both groups, no significant changes were detected.The average hemoglobin concentration in erythrocytes in the main group increased significantly from 314.5±7.3 g/l to 365.4±7.1 g/l after treatment (P<0.001). In the control group, it was 313.3±6.8 g/l before treatment and 330.5±7.5 g/l after treatment, and a significant difference (P<0.05) was detected. When comparing the average hemoglobin concentration in erythrocytes after treatment between the groups, the differences were not significant.
4. Conclusions
The results obtained in this article confirm that the appointment of antianemic therapy after antibacterial treatment in women of reproductive age with iron deficiency is highly effective. In contrast, although positive changes were observed only in the group receiving iron preparations for anemia, the differences were not highly reliable. When comparing the two groups, it was noted that the control group was more reliable in the main group than in the second control group. This confirms that it is necessary to first conduct eradication therapy treatments and then recommend antianemic treatments in women of reproductive age with Helicobacter pylori anemia. In this regard, although it is shown that morphological changes in erythrocytes remain stable for a long time, some results are reliable.
References
| [1] | World Health Organization. 2008. ISBN 97892 4 159665 7 (NLM classification: WH 155). |
| [2] | Kumari R., Bharti R.K., Singh K. et al. Prevalence of iron deficiency andiron deficiency anaemia in adolescent girls in a tertiary care hospital. J ClinDiagn Res. 2017; 11(8): BC04-BC06. DOI: 10.7860/JCDR/2017/26163.10325. |
| [3] | Chaparro C.M., Suchdev P.S. Anemia epidemiology, pathophysiology, and etiology in low- and middle-income countries. Ann N Y Acad Sci. 2019; 1450(1): 15–31. DOI: 10.1111/nyas.14092. |
| [4] | Camaschella C. Iron deficiency. Blood. 2019; 133: 30–39. DOI: 10.1182/blood-2018-05-815944. |
| [5] | Cappellini M.D., Musallam K.M., Taher A.T. Iron deficiency anaemiarevisited. J Intern Med. 2020; 287(2): 153–170. DOI: 10.1111/joim.13004. |
| [6] | Camaschella C. Iron deficiency. Blood. 2019;133:30–39. DOI: 10.1182/blood-2018-05-815944. |
| [7] | Lopez A., Cacoub P., Macdougall I.C., Peyrin-Biroulet L. Iron deficiencyanaemia. Lancet. 2016; 387(10021): 907–916. DOI: 10.1016/S0140-6736 (15)60865-0. |
| [8] | Stuklov N.I. Iron deficiency syndromes in questions and answers. Iron deficiency, anemia and pregnancy: a hematologist’s view. StatusPraesens. 2017; 5(42): 136–140. |
| [9] | Bencaiova G., Burkhardt T., Breymann C. Anemia-prevalence and riskfactors in pregnancy. Eur J Intern Med. 2012; 23(6): 529–533. DOI: 10.1016/j.ejim.2012.04.008. |
| [10] | Milman N., Taylor C.L., Merkel J., Brannon P.M. Iron status in pregnantwomen and women of reproductive age in Europe. Am J Clin Nutr. 2017; 106 (Suppl 6): 1655S–1662S. DOI: 10.3945/ajcn.117.156000. |
| [11] | Demerdash DM, Ibrahim H, Hassan DM, Moustafa H, Tawfik NM: Helicobacter pylori associated to unexplained or refractory iron deficiency anemia: an Egyptian single-center experience. Hematol Transfus Cell Ther. 2018, 40: 219-25. 10.1016/j.htct.2018.02.001. |
| [12] | Rothenbacher D., Brenner H. Burden of H. pylori and diseases in developed countries; recent developments and future implications // Microb. Infect. – 2003. –Vol. 8. – N 5. – P. 693–703. |
| [13] | Frenck. R., Clemens J. Helicobacter in the developing world // Microb. Infect. – 2003. – Vol. 8. – N 5.–P. 705–713. doi: 10.4103/1319-3767.54743. |
| [14] | Vshivkov V.A. Population epidemiology of Helicobacter pylori infection. state of the problem in Siberia. Federal State Budgetary Institution “Research Institute of Medical Problems of the North” SB RAMS, Krasnoyarsk. // Transbaikal Medical Bulletin. - 2014 - No. 2. P. 126-133. |
| [15] | Mavlyanov I.R., Nurbayev F.E., Omonov O.Y. “COVID-19 kasalligining kechishida bemorlar yosh chegarasining ahamiyati” TIBBIYOT VA SPORT jurnali 2024#4 ISSN2181-998X 142-144 bet. |
| [16] | Nurbaev F.E., Khamroev E.E. Analysis of clinical indicators of elderly and elderly patients diagnosed with chronic heart failure // African Journal of Biological Sciences. - South Africa, 2024. - No 6(10). - P. 7305-7310. |
| [17] | Nurbayev F.E. Clinical changes in the gastrointestinal system as a result of the influence COVID-19 // BIO Web of Conference 121, 04005 (2024) GLSBIA 2024 (Scopus). |
| [18] | Nurbayev F.E. Kodirova M.N. Significance of ferrokinetic spectrum indicators in iron deficiency anemia // EUROPEAN JOURNAL OF MODERN MEDICINE AND PRACTICE Vol. 4 No. 5 (May-2024) EJMMP ISSN:2795-921 X (284-289). |
| [19] | Ozdil K., Sahin A., Kahraman R. [et al.] / Current prevalence of intestinal metaplasia and Helicobacter pylori infection in dyspeptic adult patients from Turkey // Hepatogastroenterology. – 2010. – Vol. 57, № 104. – P. 1563–1566. |
| [20] | Tutar E., Ertem D., Kotiloglu Karaa E. [et al.] / Endoscopic and histopathologic findings associated with H. Pylori infection in very young children // Dig. Dis. Sci. – 2009. – Vol. 54, № 1. – P. 111–117. |
| [21] | Ozen, A. Natural history and symptomatology of Helicobacter pylori in childhood and factors determining the epidemiology of infection / A. Ozen, D. Ertem, E. Pehlivanoglu // J. Pediatr. Gastroenterol. Nutr. – 2006. – Vol. 42, № 4. – P. 398–404. |
| [22] | Santos I. S., Sassi R. A., Minten G. C. [et al.] // Validity of an epidemiologic instrument for H. Pylori screening among dyspeptic patients // Rev. Saude Publica. – 2009. – Vol. 43, № 4. – P. 639–646. |






 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML