-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(6): 2005-2010
doi:10.5923/j.ajmms.20251506.84
Received: May 28, 2025; Accepted: Jun. 15, 2025; Published: Jun. 23, 2025

Modern Concepts in the Etiology, Classification, and Surgical Management of Ventral Hernias
Khamid Yakubovich Karimov1, Murodjon Yokubjonovich Ibrokhimov2, Nargiza Aminovna Alimukhamedova3, Bobir Ibragimovich Shukurov4
1Doctor of Medical Sciences, Professor, Head of the Department of Molecular Medicine and Cell Technologies, Republican Specialized Scientific and Practical Medical Center of Hematology, Tashkent, Uzbekistan
2Surgeon, Central Polyclinic of JSC "Uzbekistan Railways", Tashkent, Uzbekistan
3Chief Physician, Central Polyclinic of JSC "Uzbekistan Railways", Tashkent, Uzbekistan
4Consulting Surgeon, Central Polyclinic of JSC "Uzbekistan Railways", Tashkent, Uzbekistan
Correspondence to: Bobir Ibragimovich Shukurov, Consulting Surgeon, Central Polyclinic of JSC "Uzbekistan Railways", Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Ventral hernias are among the most common conditions in abdominal surgery, associated with high rates of postoperative complications and recurrences. This review summarizes contemporary insights into the pathogenesis, classifications of ventral hernias (EHS, VHWG, HPW, CeDAR), approaches to assessing loss of domain, and patient risk stratification. Modern principles of surgical decision-making are discussed, including the use of various mesh implant positions and materials, as well as the importance of preoperative optimization of modifiable risk factors. Particular emphasis is placed on the advances in minimally invasive and robotic techniques, which represent the most promising directions for improving outcomes in ventral hernia repair.
Keywords: Ventral hernias, Incisional hernias, Hernia pathogenesis, Hernia classification, Loss of domain, Hernia repair, Mesh implants, Minimally invasive surgery, Complication prevention, Anterior abdominal wall surgery
Cite this paper: Khamid Yakubovich Karimov, Murodjon Yokubjonovich Ibrokhimov, Nargiza Aminovna Alimukhamedova, Bobir Ibragimovich Shukurov, Modern Concepts in the Etiology, Classification, and Surgical Management of Ventral Hernias, American Journal of Medicine and Medical Sciences, Vol. 15 No. 6, 2025, pp. 2005-2010. doi: 10.5923/j.ajmms.20251506.84.
Article Outline
1. Introduction
- Ventral hernias are among the most frequently performed surgical procedures worldwide [1]. Inguinal hernias are the most common, affecting up to 43% of men and 6% of women, and account for over 20 million surgical repairs annually [1]. In the United States alone, more than 350,000 ventral hernia repairs are performed each year, yet the failure rate remains high, contributing to increased rates of postoperative complications, hernia recurrence, and the need for reoperations [2-4]. The technical complexity of hernia correction contributes to a wide range of postoperative complications (4% to 48%) and recurrence rates up to 50%, depending on the follow-up period [5].Postoperative ventral hernias are recognized as one of the most common late complications of abdominal surgery. It is estimated that up to 3% of patients develop symptomatic hernias within two years after open abdominal procedures [6]. Reoperative surgeries further increase the risk of recurrence and complications [7]. These events also impose a considerable financial burden on healthcare systems [8], particularly in patients with modifiable risk factors such as smoking, obesity, and diabetes mellitus [9].For example, in Japan, the average annual cost of treating postoperative ventral hernias is estimated at $1.7 billion [10]. Thus, even a 1% reduction in surgical interventions could save approximately $17 million annually for the Japanese healthcare system [10]. Similarly, the French Surgical Society estimates that reducing the incidence of postoperative hernias by at least 5% annually could save up to €4 million each year [11].Despite numerous studies, the multifactorial nature of ventral hernia pathogenesis remains poorly understood. While clinical risk factors such as obesity, diabetes, aging, male sex, smoking, as well as surgical factors like suture type and closure technique, are well established [2,13], they only offer a partial understanding of the overall risk.
2. Definitions and Classification of Ventral Hernias
- The primary aim of hernia classification systems is to establish a standardized and universally accepted terminology that facilitates data comparison across studies and supports surgical decision-making. Effective classification must consider various parameters, including hernia characteristics and patient condition.The European Hernia Society (EHS) classification is one of the most widely accepted systems for describing both primary and incisional anterior abdominal wall hernias [13]. A primary hernia is defined as a spontaneous protrusion of intra-abdominal contents through a defect in the abdominal wall, unrelated to previous surgery or trauma. The EHS system classifies hernias based on two parameters: location and defect size.Regarding location, two types of midline hernias (epigastric and umbilical) and two types of lateral hernias (Spigelian and lumbar) are recognized. Defect size is measured by the maximal transverse diameter. According to joint EHS and American Hernia Society (AHS) guidelines, the size classification is as follows: small (<1 cm), medium (1–4 cm), and large (>4 cm) for midline hernias [14]. For lateral hernias, the thresholds are slightly different: small (<2 cm), medium (2–4 cm), and large (>4 cm) [13].An incisional hernia is defined as any defect in the anterior abdominal wall, with or without protrusion, located in the area of a previous surgical scar and detectable on clinical examination or imaging [15]. This category also includes primary hernias previously treated surgically. The EHS consensus emphasizes two key parameters for classifying incisional hernias: location and size [16].Several systems have been developed to predict postoperative outcomes and risk of complications in ventral hernia repair:1. Ventral Hernia Working Group (VHWG) ClassificationFirst introduced in 2010 and revised in 2013, the VHWG classification stratifies the risk of surgical site complications and hernia recurrence [17,18]. It includes:- Grade 1 (low risk): healthy patients without prior wound infections.- Grade 2 (comorbid): patients with conditions such as diabetes, COPD, immunosuppression, obesity, or smoking.- Grade 3 (contaminated field): presence of surgical field contamination.ο 3A: clean-contaminated;ο 3B: contaminated;ο 3C: active infection.While primarily designed for open surgery, its applicability in minimally invasive procedures is less evident, given the reduced infection rates in such cases [19].2. The Hernia–Patient–Wound (HPW) classification, developed by Petro and Novitsky in 2016, is analogous to the TNM cancer staging system and offers a stratified prediction of postoperative complications and recurrence risk [20].- H (hernia):ο H1: <10 cmο H2: 10–20 cmο H3: >20 cm- P (patient comorbidity):ο P0: noneο P1: obesity (BMI >35), smoking, diabetes, immunosuppression- W (wound):ο W0: cleanο W1: contaminatedFor instance, HPW stage 4 (H3 P1 W1) corresponds to a 39% risk of early complications and 31% risk of recurrence, whereas HPW stage 1 (H1 P0 W0) corresponds to only 6% and 5%, respectively [20] (Fig. 1).
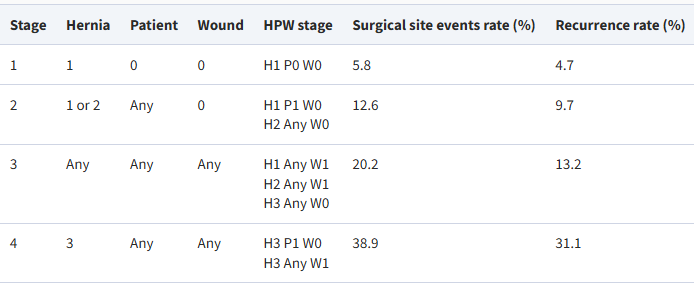 | Figure 1. Classification of Hernia–Patient–Wound (HPW) for predicting complications after hernioplasty [20] |
3. Surgical Treatment Approaches
- The rapid evolution of ventral hernia repair techniques has highlighted the need for standardized terminology, particularly regarding mesh placement planes. Terms such as sublay and underlay have historically been used inconsistently, referring to various anatomical positions. In 2020, an international panel of 20 experts conducted a Delphi consensus to propose standardized nomenclature for mesh positioning within the anterior abdominal wall.The proposed anatomical planes include:- Onlay – placed on top of the anterior fascia;- Anterectus – between the anterior rectus sheath and the rectus muscle;- Inlay – sutured to the edges of the defect without overlap;- Interoblique – between the external and internal oblique muscles;- Retro-oblique – below the internal oblique and above the transversus abdominis;- Retrorectus – between the rectus muscle and its posterior sheath;- Retromuscular (post-TAR) – retrorectus medially, between the transversus abdominis and its fascia laterally;- Transversalis fascial – between the posterior sheath and transversus abdominis;- Preperitoneal – between the transversalis fascia and peritoneum;- Intraperitoneal – beneath the peritoneum and in contact with abdominal organs.Among these, onlay, retrorectus, retromuscular, preperitoneal, and intraperitoneal placements are the most commonly used in clinical practice.A wide variety of mesh implants is available for hernia repair. A useful classification system groups them by material composition. In 2018, a standardized mesh labeling system was proposed that distinguishes between: permanent synthetic, biologic, bioabsorbable (synthetic absorbable), and hybrid meshes.Meshes are also categorized by pore size (microporous <100 μm, small 101–600 μm, medium 601–1000 μm, large 1001–2000 μm, extra-large >2000 μm), weight (ultralight <35 g/m², light 35–50 g/m², medium 51–90 g/m², heavyweight >90 g/m²), and material type. Although no definitive correlation has been established between these properties and outcomes, current evidence suggests that lightweight, medium-porosity meshes provide better integration and reduced foreign body sensation.In clean, non-contaminated cases, permanent synthetic meshes are preferred. In contaminated fields, the choice remains controversial. Some data support the use of synthetic mesh even in contaminated environments, although long-term outcomes remain uncertain. Common synthetic materials include polypropylene, polyester, polyvinylidene fluoride (PVDF), and expanded polytetrafluoroethylene (ePTFE). Among these, polypropylene meshes remain the most widely studied and utilized.Biologic meshes, derived from human or animal tissue, are used primarily in infected or high-risk wounds. However, randomized trials have not demonstrated clear superiority over synthetic options in complex scenarios.Bioabsorbable meshes, composed of fully or partially degradable polymers, provide a more affordable alternative to biologics. Their degradation time ranges from a few months to 1–1.5 years. Hybrid meshes, which combine properties of synthetic and biologic materials, represent the most recent innovation, though clinical data remain limited.
4. Preoperative Optimization and Risk Modification
- For patients undergoing elective ventral hernia repair, preoperative optimization of both hernia- and patient-related modifiable risk factors is essential to improve surgical outcomes without causing undue delays in treatment. As outlined in risk classification systems, certain specific factors significantly influence the likelihood of complications.Smoking cessation is strongly recommended at least four weeks prior to surgery, irrespective of hernia type. This intervention significantly reduces the risk of wound and pulmonary complications.Obesity is one of the most impactful risk factors for both postoperative complications and recurrence. Patients with a body mass index (BMI) >30 kg/m² are at particularly high risk [7]. For those with BMI >35 kg/m², elective surgery should be approached cautiously, and preoperative weight reduction is encouraged. Recent advances in minimally invasive techniques, however, have been associated with a decline in complication rates among obese patients.In patients who are unresponsive to diet and physical activity alone, pharmacological options such as GLP-1 receptor agonists may promote significant weight loss within 3–6 months preoperatively. Bariatric surgery can also be considered either as a preparatory step or combined with hernia repair in patients with morbid obesity.Diabetic patients with poor glycemic control are at increased risk for postoperative infectious complications and recurrence. Although some studies show no clear association between HbA1c levels and adverse outcomes, most experts recommend maintaining HbA1c below 8% and ensuring tight perioperative glucose control.Ventral hernia patients often report reduced physical activity due to fear of worsening the hernia. However, prehabilitation even in the form of light individualized exercise has been shown to improve postoperative outcomes.In patients with large hernias and loss of domain, botulinum toxin type A (BTA) injections into the lateral abdominal wall muscles promote muscle relaxation and elongation, facilitating fascial closure. Typically, Botox® is administered in a dose of 100–300 IU across three sites on each side, four weeks prior to surgery, under ultrasound guidance. Adverse effects are rare and usually limited to transient pain or hematomas at injection sites. Nevertheless, the technique still requires further standardization.Progressive preoperative pneumoperitoneum (PPP) may be used as an adjunct or alternative to BTA in cases of severe loss of domain. In this technique, 800–1000 mL of gas is insufflated daily via an intra-abdominal catheter to gradually stretch the abdominal wall. While PPP can facilitate hernia content reduction, complications such as pulmonary dysfunction, thromboembolism, bowel injury, and even rare fatal outcomes have been reported.
5. Surgical Management of Primary Midline and Incisional Hernias
- Umbilical and epigastric hernias represent some of the most frequently encountered types of ventral hernias. Although their true prevalence is unknown, surgical repair is commonly performed. Patients may remain asymptomatic or report bulging, intermittent pain, or discomfort. In asymptomatic cases, a watchful waiting strategy may be appropriate, but approximately 20% of such patients eventually require operative intervention.In most cases of primary midline hernias, imaging studies are not necessary. However, for large hernias, potential loss of domain, obesity, rectus diastasis, uncertain defect size, or suspicion of multiple defects, ultrasound or computed tomography (CT) is recommended for optimal surgical planning [14].Surgical repair may be performed using open or minimally invasive approaches. The choice depends on patient characteristics, defect size and location, institutional resources, and surgeon experience.- For small defects (<1 cm), open repair is typically preferred. The decision between suture repair and mesh placement should be made on an individual basis. Mesh use has been shown to reduce recurrence even in small hernias, although it may slightly increase the risk of wound-related complications. When mesh is used, a flat preperitoneal polypropylene implant with at least 2 cm overlap is recommended [14].- For medium defects (1–4 cm), mesh reinforcement is strongly recommended. Open mesh repairs should provide a 3 cm overlap to minimize recurrence without significantly affecting wound complications or postoperative discomfort [14]. The decision between open and minimally invasive techniques should be individualized. Minimally invasive approaches are preferred in patients at high risk of wound complications or with multiple defects [14]. An additional benefit of laparoscopy is the ability to identify occult hernias and place a large mesh for linea alba weakness or diastasis recti.- Defects >4 cm are less commonly encountered in primary cases and are generally managed under the framework for incisional hernias [14].Among all ventral hernias, incisional hernias represent the most heterogeneous category in terms of size, location, and associated anatomical challenges. Their development is influenced by numerous factors, including patient-specific characteristics, initial disease pathology, incision type, and closure technique.CT imaging is frequently employed in the preoperative evaluation of incisional hernias to assess anatomy, defect size and location, presence of mesh, and to identify ongoing risk factors such as occult pathology or infection.When the defect width exceeds 8 cm or loss of domain is present, component separation techniques may be necessary to facilitate fascial closure. Proximity to bony structures can complicate mesh fixation and may require the use of bone anchors titanium or polymeric devices drilled or driven into bone to securely affix the mesh. This method is particularly useful in large incisional hernias involving the pubis, costal margins, or iliac crests.In patients with reducible, asymptomatic incisional hernias, the risk of emergent repair is low, and observation may be appropriate. However, such hernias typically enlarge over time, leading to increased technical difficulty and poorer outcomes. Patients with symptomatic hernias that impair quality of life should be offered surgery if medically fit. Where benefit is uncertain, discussions regarding potential risks and preoperative risk factor optimization are essential.
6. Trends in Open, Laparoscopic, and Robotic Repair
- Advancements in the anatomical understanding of the anterior abdominal wall have significantly modified traditional open reconstruction techniques. Among these, retromuscular (retrorectus) mesh placement remains the most durable open repair technique and is widely considered the standard for midline hernia reconstruction. However, open repairs are associated with a higher risk of postoperative wound complications and prolonged hospital stay.Minimally invasive hernia repair techniques, both laparoscopic and robotic, are increasingly being adopted for both midline and lateral incisional hernias. Over the past two decades, intraperitoneal onlay mesh (IPOM) repair with defect closure and mesh fixation has gained widespread use. Nonetheless, concerns about adhesions and chronic pain from mesh fixation have prompted the development of newer techniques that place mesh in preperitoneal or retromuscular planes.These advanced anatomical planes can be accessed via traditional laparoscopy or robotic platforms. Although these techniques are more technically demanding and time-consuming, they offer advantages such as reduced postoperative pain, shorter hospitalization, and lower infection risk, making them an attractive option for surgeons with expertise in minimally invasive techniques.
7. Conclusions
- Ventral hernias remain a significant clinical challenge due to high recurrence rates, frequent postoperative complications, and substantial healthcare costs. Modern understanding of their pathogenesis, classification, and treatment strategies continues to evolve. The implementation of standardized classification systems (EHS, VHWG, HPW, CeDAR) facilitates consistent diagnostic criteria and outcome prediction.Patient stratification based on modifiable risk factors including smoking, obesity, and diabetes—is critical. Preoperative optimization using physical conditioning, pharmacologic weight loss, botulinum toxin injection, or progressive pneumoperitoneum plays an increasingly important role.Surgical trends indicate a transition from traditional open techniques toward minimally invasive and robotic approaches, with growing emphasis on retromuscular and preperitoneal mesh positioning. The use of permanent synthetic, bioabsorbable, or hybrid meshes is expanding, although high-level evidence is still needed for newer materials.The future of ventral hernia management lies in personalized treatment planning, guided by validated risk prediction models and continuous innovation in surgical materials and technology for abdominal wall reconstruction.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML