-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(6): 1930-1932
doi:10.5923/j.ajmms.20251506.64
Received: May 13, 2025; Accepted: Jun. 12, 2025; Published: Jun. 20, 2025

Morphofunctional Changes in Salivary Glands under the Influence of Groundwater
Teshaeva Dilbar Shukhratovna
Assistant of the Department of Anatomy and Clinical Anatomy (OHTA), Bukhara State Medical Institute named after Abu Ali ibn Sina, Bukhara, Uzbekistan
Correspondence to: Teshaeva Dilbar Shukhratovna, Assistant of the Department of Anatomy and Clinical Anatomy (OHTA), Bukhara State Medical Institute named after Abu Ali ibn Sina, Bukhara, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Currently, the shortage of drinking water is becoming one of the global issues, and scientists emphasize that in the future, groundwater may become the main source of drinking water. However, direct consumption of groundwater may have negative effects on the human body due to its mineral composition, which affects many organs. The minerals present in groundwater play an important role in both general health and oral health. Salivary glands are vital to the body, with their main function being the secretion of saliva into the oral cavity. The composition and secretion of saliva support various physiological functions essential for maintaining overall health. This article presents changes in the major salivary glands caused by groundwater consumption, along with the results of biochemical and immunological studies of saliva. Research objective: To study the effect of groundwater on the major salivary glands.
Keywords: Immunological, Interleukin, pH, Water hardness, Groundwater, White rat
Cite this paper: Teshaeva Dilbar Shukhratovna, Morphofunctional Changes in Salivary Glands under the Influence of Groundwater, American Journal of Medicine and Medical Sciences, Vol. 15 No. 6, 2025, pp. 1930-1932. doi: 10.5923/j.ajmms.20251506.64.
1. Introduction
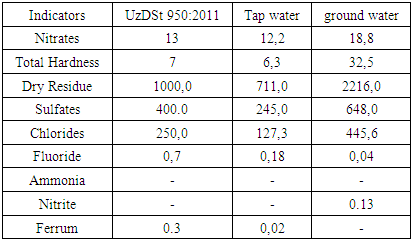
- It is well known that the scarcity of drinking water is becoming an increasingly pressing global concern. Scientists emphasize the utilization of groundwater as a potential source of potable water. [5,13] However, the composition of drinking water can differ significantly from that of treated tap water. In particular, the use of saline or mineral-rich groundwater as a drinking source has been associated with various physiological changes in the human body. [6,14] Continuous consumption of water with distinct physicochemical properties may also affect the salivary glands and, consequently, the composition and function of saliva. [8,9] It is established that many diseases in the human body originate from external factors. Every substance that enters the body from the environment—especially via the digestive tract—exerts both direct and indirect influences on physiological systems. When such influences are negative and persist over time, they can result in serious pathological changes. While the impact of groundwater on certain organs—such as the thyroid gland—has been previously studied, its potential effects on the salivary glands remain largely unexplored [10,12]. This research gap highlights the urgent need for further investigation and underscores the relevance of the topic.The oral cavity is often referred to as the "mirror" of the body, and the salivary glands play a critical role in maintaining oral and systemic health. [9,11,15] In addition to initiating digestion, saliva protects the oral mucosa and teeth, combats microbial invasion, and helps regulate the oral pH balance. Impaired salivary gland function or reduced saliva production can lead to a range of health complications. Therefore, preserving the health of the salivary glands is essential for overall physiological well-being. [3,4]Objectives:1. To analyze the chemical composition of groundwater extracted from a depth of 10 meters on the territory of the Bukhara State Medical Institute and compare it with tap water and the standards set by O’zDSt 950:2011.2. To identify morphological changes in the salivary glands of healthy white laboratory rats (6–9 months old, 250–300 g) consuming groundwater.3. To evaluate the immunological composition of saliva in rats over a three-month period and compare results between those consuming groundwater and those consuming tap water.
2. Materials and Methods
- For this study, healthy, non-breed-specific white rats weighing 250–300 grams were used as experimental subjects. A total of 130 white laboratory rats were divided into two experimental groups. The first group, comprising 50 rats, consumed centralized (tap) drinking water. The second group, consisting of 80 rats, was provided with groundwater extracted from a depth of 10 meters.The rats were divided into the following two groups:Group I (Control Group): These laboratory rats consumed centralized tap water according to municipal standards. The water was administered daily for a period of three months.Group II (Experimental Group): These rats consumed groundwater sourced from a depth of 10 meters below the surface. The groundwater was administered daily over a three-month period.Below, the composition standards for potable water as established by the Uzbekistan State Standards in 2011 are provided. Additionally, the physicochemical characteristics of the groundwater used in this study—sourced from the Bukhara State Medical Institute—are compared to the composition of the municipal tap water used.
3. Results and Discussion
|
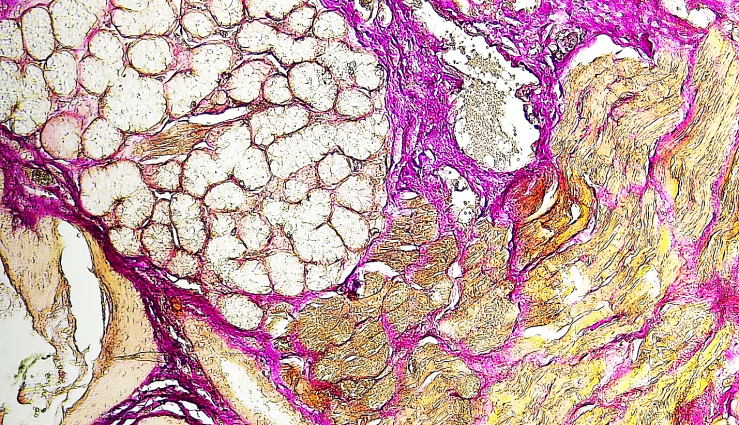 | Figure 1. Histological description: sublingual salivary gland. Hematoxylin and eosin staining. Magnification 10×4 |
|
4. Conclusions
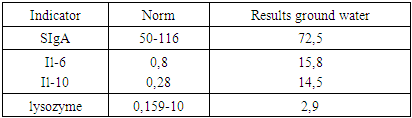
- 1. The groundwater used in this study exceeds several parameters outlined in O’zDSt 950:2011 and requires treatment before being used as drinking water.2. Groundwater consumption in rats led to morphological changes in the parotid salivary glands, including cellular hypertrophy and edema.3. Immunological analysis of saliva demonstrated inflammatory activation, weakened immune defense, and decreased antimicrobial protection.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML