Djabbarova Nasiba Yusupovna, Khodjieva Dilbar Tajievna
Bukhara State Medical Institute, Bukhara, Uzbekistan
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
This article discusses the clinical, epidemiological, neurological, and immunological features of myasthenia gravis, one of the most pressing problems in neurology. To date, no work has been carried out in Uzbekistan on the epidemiology and immunological mechanisms of myasthenia gravis. Defining the epidemiological indicator of patients with myasthenia gravis, studying the clinical-epidemiological, neurological, and immunological characteristics will undoubtedly be an important step in finding a solution to this urgent problem. The need to develop a unified approach to the management of patients with myasthenia gravis served as the basis for choosing the topic of this study.
Keywords:
Myasthenia gravis, Immunology, Epidemiology, Muscles, Neurophysiology
Cite this paper: Djabbarova Nasiba Yusupovna, Khodjieva Dilbar Tajievna, Clinical, Epidemiological, Neurological, and Immunological Characteristics of Myasthenia, American Journal of Medicine and Medical Sciences, Vol. 15 No. 6, 2025, pp. 1868-1872. doi: 10.5923/j.ajmms.20251506.49.
1. Relevance
Myasthenia gravis is a chronic autoimmune disease that clinically manifests as weakness and rapid fatigue in striated muscles. In the pathogenesis of myasthenia gravis, there is an autoimmune attack on postsynaptic acetylcholine receptors and other protein systems of the neuromuscular synapse, as a result of which neuromuscular conductivity is disrupted [2,5,8]. Since the disease mainly occurs in young people with a progressive or wave-like course, mainly in able-bodied people, patients experience a decrease in work capacity and quality of life, leading to serious medical and social problems. Myasthenia gravis has different epidemiological indicators in different countries, ranging from 2.17 to 32 cases per 100,000 population [1,4,10]. Different epidemiological indicators showed different prevalence and mortality rates in different regions. In the last decade, various authors have noted an increase in morbidity rates in all age groups. Despite the high prevalence of the disease in young people, recent data indicate that the disease can begin in those over 50 years of age, especially in the elderly. Nevertheless, there are not many works aimed at studying the epidemiology of myasthenia gravis [3,6,9,11]. In the conditions of Uzbekistan, accurate epidemiological data are important for the population to receive specialized medical care and improve the quality of diagnosis and treatment of myasthenia gravis. The diversity of clinical manifestations at the onset of the disease, their individual characteristics in different age groups and sex, and the course of the disease create difficulties in diagnosing myasthenia gravis [3,7,12]. To date, a number of antibodies have been identified, which indicates the relevance of determining the clinical features in patients due to the high heterogeneity of the disease.Research objective. Study of clinical-epidemiological, neurological, immunological, and gender characteristics of myasthenia gravis, optimization of treatment.
2. Materials and Methods of Research
For a comprehensive assessment of the clinical picture of the disease, the neurological status and laboratory parameters of patients were assessed. In order to objectively assess the neuromuscular conduction block, a proserin test was performed. In the study, a MSCT examination of the chest was performed to identify myasthenia gravis associated with thymoma. MSCT of the thoracic organs was performed on a Siemens Somatom Definition AS device in the 70-150 kV range at 1 mm. All patients underwent general blood, general urine tests, blood clotting time, and biochemical blood tests. Determination of the concentration of antibodies to the acetylcholine receptor and antibodies to the MUSC was carried out by enzyme-linked immunosorbent assay using the diagnostic test system Bio Vendor Laboratory Medicine, Inc., Acetylcholine Receptor Autoantibody ELISA (Czech Republic). The titer of antibodies to the acetylcholine receptor was assessed as high when it exceeded 0.5 nmol/l. Statistical processing of the research results was carried out using parametric and nonparametric methods using the application software package of the personal computer "IBM SPSS Statistics v.26" (manufacturer - IBM Corporation). All the obtained data were analyzed using Microsoft Office Excel 2016 spreadsheets. The indicated statistical indicators for the amount of assessed data are the absolute and relative number of patients, the mean value, and standard deviations. Quantitative indicators were assessed according to the Shapiro-Wilka criterion (when the number of examiners exceeded 50) or according to the Kolmogorov-Smirnov method (when the number of examiners was less than 50). The indicators of asymmetry and excess were also assessed. The data obtained during the assessment of quantitative indicators were combined in a variation series, the arithmetic mean (M), standard deviation (SD), standard error (m), and 95% confidence interval (95% CI) were estimated.
3. Results
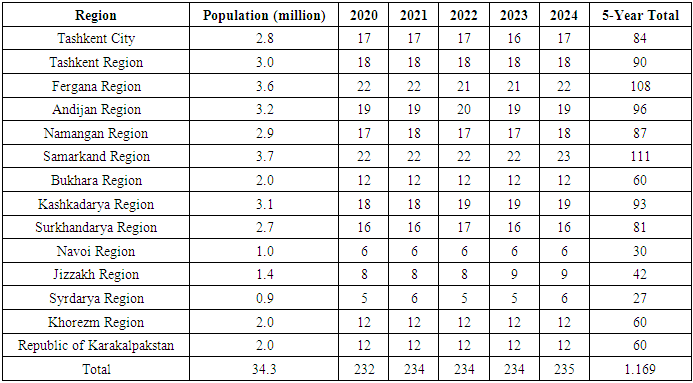
A total of 120 patients were analyzed, divided into two groups: with thymoma and without thymoma. Also, patients in each group were indicated separately by gender. The number of patients without thymoma in the study was 68 (56.67±4.52%), of which 41 (34.17±4.33%) were men and 27 (22.5±3.81%) were women. The number of patients with thymoma was 52 (43.33±4.52%), including 26 men (21.67±3.76%) and 26 women (21.67±3.76%).Table 1. Estimated cases of myasthenia gravis in Uzbekistan (2020-2024, by region)
 |
| |
|
Analysis of estimated cases of myasthenia gravis in Uzbekistan for 2020-2024 shows that this neuromuscular disease is not spread at the same intensity throughout the country, and there are certain differences between regions. According to the data in the table, approximately 1169 new cases may have been registered in the republic over five years, which was determined on average at the rate of 3 cases/ 100,000 population, depending on the population size.The highest rates of the disease were recorded in Samarkand (111), Fergana (108), Tashkent region (90) and the city of Tashkent (84). This may be due to high population density in these areas, better access to medical services, as well as the activity of neurologists. On the other hand, relatively few cases were recorded in Syrdarya (27), Navoi (30), and Jizzakh (42) regions, which can be explained by either low population size or limited diagnostic capabilities. Over the years, there has been no significant change in the number of new cases: 232 cases in 2020, and 235 cases in 2024. Such stability, on the one hand, is characteristic of the epidemiological nature of the disease, and on the other hand, is associated with the use of a constant coefficient in approximate modeling. Also, the data may differ from the actual clinical picture, since they are based on demographic estimates, and not on specifically diagnosed cases of patients. The results of the analysis show that it is necessary to form a clear and complete epidemiological list of relatively rare autoimmune diseases, such as myasthenia gravis, to create a special registry at the republican level for their monitoring and correct diagnosis at the regional level. It is also advisable to develop strategies aimed at increasing awareness, early diagnosis, and treatment among medical workers and the population in areas with low indicators. Table 2 presents the distribution of patients according to the extended Osserman classification (types I, IIa, IIb, III, IV), which assesses the degree of clinical severity in patients with myasthenia gravis. Differences between groups depending on the presence or absence of thymoma were assessed using χ2 statistical tests. This detailed form of the Osserman classification is an important clinical instrument in determining the pathogenesis of the disease, response to treatment, and prognosis.Table 2. Distribution of myasthenia gravis by extended Osserman types depending on the presence of thymoma and statistical differences (M±m)
 |
| |
|
Type I (focal form): The incidence of type I among patients with thymoma was 26.92±6.15%, while in the group without thymoma it was 19.12±4.77%. Among patients with this form, thymomatous changes were recorded relatively more often. Type IIa (most mildly generalized form): almost equally and to a low degree in both groups - 13.24±4.11% in those without thymoma and 15.38±5% in those with thymoma. This clinical variant does not have a significant correlation with the presence of thymoma. Type IIb (moderate generalized form): significantly higher among patients without thymoma (27.94±5.44%), and very low among patients with thymoma (3.85±2.67%). This suggests that type IIb myasthenia gravis may be associated with the absence of thymoma. This difference was assessed as statistically significant with indicators χ2 = 11.269; p = 0.024.Type III (severe generalized form): 28.85±6.28% in patients with thymoma and 14.71±4.29% without thymoma. This tendency indicates a pathogenetic link between clinically severe forms of the disease and the presence of thymoma.Type IV (late-onset severe form): The distribution in this form was proportional and equal (25%) in both groups, and the effect of thymoma was not noted. According to the results of Pearson's χ2 test, there is a statistically significant difference in the overall distribution (χ2 = 13.498; p = 0.009), especially differences in the sections of types IIb and III were noted as the main factors shaping the result.Thus, significant clinical differences are formed by the fact that type IIb of myasthenia gravis is preferable in the absence of thymoma, and type III, on the contrary, is more often noted in patients with thymoma. This can serve as an important criterion in the development of personalized treatment and prognosis models, confirming the presence of a pathogenetic relationship between trends, thymomatous changes, and clinical forms of myasthenia gravis.Table 3. Distribution of myasthenia gravis by MGFA severity and remission states depending on the presence of thymoma (M±m)
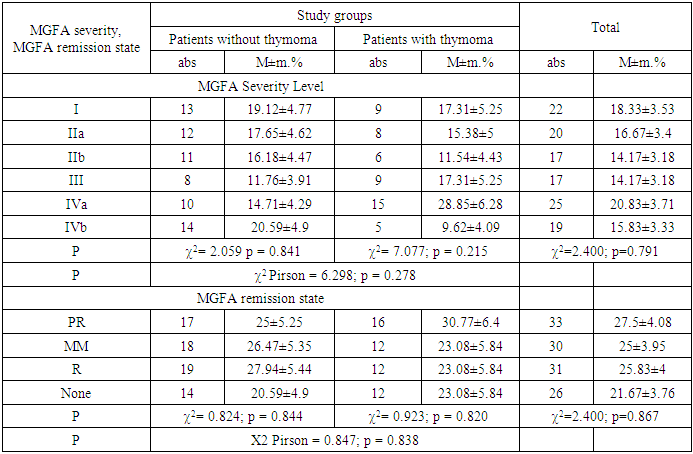 |
| |
|
A comparative analysis of the degree of clinical severity and remission of patients with myasthenia gravis according to the MGFA (Myasthenia Gravis Foundation of America) classification in relation to the presence of thymoma was conducted, and the MGFA system is an important criterion for systematic assessment of the disease and individualization of treatment tactics in global clinical practice.Analysis of MGFA by weight gradations showed that patients with grade I in the group without thymoma were 19.12±4.77%, and in the group with thymoma - 17.31±5.25%. A similar trend was observed at levels IIa and IIb, with practically similar indicators recorded in both groups (for example, at IIb: 16.18% no thymoma, 11.54% thymoma). This means that there is no direct correlation between the severity of the pathogenic process and thymomatous changes.A high frequency of cases was noted in patients with thymoma forms III and IVa (III: 17.31% and 11.76%; IV a: 28.85% and 14.71%), which implies a possible pathogenic connection of thymomatous pathology with the severity of the disease. Nevertheless, the overall distribution did not have a statistically significant difference (χ2 Pirson = 6.298; p = 0.278), which limits the possibility of considering the structure of clinical severity levels depending on the presence of thymoma as a general basis.Analysis of the MGFA remission standards PR (Partial Remission), MM (Minimal Manifestation), R (Pharmacologic Remission), and None (no remission) showed a proportional distribution of remission cases in both groups.It was found that although the PR status was somewhat higher (30.77%) in the group with thymoma, the differences between the groups in MM and R cases were insignificant. The proportion of patients who did not achieve remission was also proportional (thymoma absent: 20.59%; thymoma present: 23.08%). Differences based on χ2 tests in all groups did not reach the level of statistical significance (χ2 Pearson = 0.847; p = 0.838), which indicates a limited influence of thymoma on the state of remission.Thus, the distribution of MGFA severity and remission cases is formed independently of the presence or absence of thymoma, and thymomatous changes cannot be considered as the main moderator influencing the clinical course, response to treatment, and prognosis. At the same time, the differences observed in certain clinical classes (in particular, at the IVa level) do not exclude the presence of a potential pathogenic association and require additional studies in specific subgroups.Table 4. Dynamics of MG-ADL, CMG, and MG-QOL15 indicators in patients with myasthenia gravis depending on the presence of thymoma (M±m)
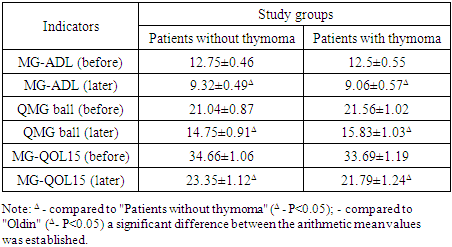 |
| |
|
In this table, the dynamics of MG-ADL (Myasthenia Gravis Activities of Daily Living), QMG (Quantitative Myasthenia Gravis Score), and MG-QOL15 (Myasthenia Gravis Quality of Life 15) indicators in patients with myasthenia gravis before and after treatment were assessed depending on the presence or absence of thymomatous changes. This approach allows for the determination of the effectiveness of clinical intervention. According to the MG-ADL indicator, which assesses the level of functional activity of patients, the average value in patients without thymoma before treatment was 12.75±0.46 points, and in patients with thymoma - 12.5±0.55 points. The difference between the groups is not statistically significant. After treatment, a significant decrease in this indicator was noted in both groups (9.32±0.49 in patients without thymoma; Δ), which indicates a decrease in symptoms to a clinically significant level.According to the KMG indicator, which assesses the objective clinical severity of the disease, 21.04±0.87 points were determined in patients without thymoma in the initial stage, 21.56±1.02 points in patients with thymoma. After treatment, the indicators decreased proportionally, reaching 14.75±0.91 and 15.83±1.03 points, respectively, demonstrating a decrease in the severity of the disease. The changes, denoted by Δ, were assessed as statistically significant.A similar dynamic was observed in the MG-QOL15 indicator, reflecting the level of patients' quality of life: before treatment, it was 34.66±1.06 points for those without thymoma and 33.69±1.19 points for those with thymoma. After therapeutic intervention, these indicators significantly decreased and amounted to 23.35±1.12 and 21.79±1.24 points, respectively (Δ), which confirms a trend towards improvement in the quality of life.
4. Conclusions
Thus, the results of the study, by analyzing the dynamics of MG-ADL, CMG, and MG-QOL15 indicators, proved the effectiveness of therapeutic intervention in myasthenia gravis despite the presence of thymoma. The changes noted during the study were statistically significant, with significant improvements in functional status, disease severity, and quality of life. The fact that the presence of thymomatous changes did not cause certain differences in the level of therapeutic response indicates that it is not necessary to consider it as the main moderator in assessing these clinical parameters. However, in-depth analysis of subgroups in the context of dynamic changes allows for further identification of pathogenetic and prognostic relationships.
References
| [1] | Andersen, J.B. Myasthenia gravis epidemiology in a national cohort; combining multiple disease registries / J.B. Andersen, A.T. Heldal, A. Engeland [et al.] // Acta Neurologica Scandinavica. – 2014. – Vol. 129. – P. 26-31. |
| [2] | Aragones JM, Altimiras J, Roura P. Prevalence of myasthenia gravis in the Catalan country of Osona. Neurologia. 2017; 32(1): 1-5. |
| [3] | Ayres, A. Cognitive performance in patients with Myasthenia Gravis: an association with glucocorticosteroid use and depression / A. Ayres P.B. Winckler L.A. Jacinto-Scudeiro [et al.] // Dementia & Neuropsychologia. – 2020. – Vol. 14. – P. 315- 323. |
| [4] | Balestra B., Moretti M., Longhi R., Mantegazza R., Clementi F., Gotti С. Antibodies against neuronal nicotinic receptor subtypes in neurological disorders // J. Neuroimmunol. - 2000. - V. 102, № 1. - P. 89-97. |
| [5] | Ballard, C. Efficacy, safety and tolerability of rivastigmine capsules in patients with probable vascular dementia: the Vantage study / C. Ballard, M. Sauter, P. Scheltens [et al.] // Current medical research and opinion. – 2008. – Vol. 24, № 9. – P. 2561-2574. |
| [6] | Binks S, Vincent A, Palace J. Myasthenia gravis: a clinical-immunological update. J Neurol. 2016; 263(4): 826-834. DOI: 10.1007/s00415-015-7963-5. |
| [7] | Caress, J.B. Anti-MuSK myasthenia gravis presenting with purely ocular findings / J.B. Caress, C.H. Hunt, S.D. Batish / J.B. Caress // Arch Neurol. – 2005. – Vol. 62, № 6. – P. 1002-1003. |
| [8] | Ceremuga, T.E. Etiology, mechanisms, and anesthesia implications of autoimmune myasthenia gravis / T.E. Ceremuga, X.L. Yao, J.T. McCabe [et al.] // AANA J. – 2002. – Vol. 70, № 4. – P. 301-310. |
| [9] | Diaz-Manera, J. Treatment strategies for myasthenia gravis / J. Diaz– Manera, R. Rojas–Garcia, I. Illa [et al.] // Expert Opin Pharmacother. – 2009. – Vol. 8, № 10. – P.1329-1342. |
| [10] | Eizaguirre, M.B. Neuropsychological performance in patients with myasthenia gravis / M.B. Eizaguirre, F. Aguirre, C. Yastremiz [et al.] // Medicina. – 2017. – Vol. 77(2). – P. 117-120. |
| [11] | Gilhus, N.E. Myasthenia gravis-autoantibody characteristics and their implications for therapy / N.E. Gilhus, G.O. Skeie, F. Romi [et al.] // Nature reviews neurology. – 2016. – Vol. 12, № 5. – P. 259-268. |
| [12] | Hellmann MA, Mosberg-Galili R, Steiner I. Myasthenia gravis in the elderly. J Neurol Sci. 2013; 325(1-2): 1-5. |


 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML


