-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(6): 1864-1867
doi:10.5923/j.ajmms.20251506.48
Received: May 21, 2025; Accepted: Jun. 12, 2025; Published: Jun. 14, 2025

Prediction and Prevention of Acute Ischemic Stroke in Patients with Indication for Coronary Artery Bypass Grafting
H. M. Tursunov, M. M. Bakhadirkhanov, S. N. Gulomitdinov
Republican Research Center of Emergency Medicine, Tashkent, Uzbekistan
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The aim of the study was to develop a system for predicting the risk of acute ischemic stroke in patients with indications for coronary artery bypass grafting and to determine the optimal methods of prevention of this complication. Background. Coronary artery bypass grafting is one of the most efficient methods for surgical treatment of coronary heart disease. Despite the improvement of surgical technique and anesthesiology, the incidence of postoperative neurological complications, particularly acute ischemic stroke, remains a significant problem in cardiac surgery, ranging from 1.5% to 5.2%. Material and methods. The data of 30 patients (22 men, 8 women, mean age 68.7±7.2 years) with development of acute ischemic stroke after coronary artery bypass grafting were analyzed. A comprehensive preoperative examination including neuroimaging and ultrasound duplex scanning of brachiocephalic arteries were performed. Multivariable logistic regression analysis with development of a predictive model was used to identify risk factors. Results. Stroke was developed on an average of 2.4±1.7 days after surgery. Independent predictors identified were as follows: age >70 years (OR 2.8), brachiocephalic artery stenosis >70% (OR 3.4), atherosclerosis of the ascending aorta (OR 3.2), atrial fibrillation (OR 2.5), diabetes mellitus (OR 1.9), artificial circulation time >120 min (OR 2.3), and intraoperative hypotension (OR 2.1). The predictive model showed high informative power (AUC 0.85, sensitivity 79.3%, specificity 81.6%). A complex of preventive measures in high-risk patients reduced the incidence of stroke by 47%. Conclusion. A personalized approach to risk stratification and prevention of acute ischemic stroke can be efficiently integrated into clinical protocols for cardiac surgery patients to improve patient outcomes.
Keywords: Coronary artery bypass grafting, Acute ischemic stroke, Risk factors, Prognosis, Prevention
Cite this paper: H. M. Tursunov, M. M. Bakhadirkhanov, S. N. Gulomitdinov, Prediction and Prevention of Acute Ischemic Stroke in Patients with Indication for Coronary Artery Bypass Grafting, American Journal of Medicine and Medical Sciences, Vol. 15 No. 6, 2025, pp. 1864-1867. doi: 10.5923/j.ajmms.20251506.48.
Article Outline
1. Introduction
- Coronary artery bypass grafting (CABG) is one of the most efficient methods for surgical treatment of coronary heart disease (CHD) [11]. Despite the improvement of surgical technique and anesthesiology, the incidence of postoperative neurological complications, particularly acute ischemic stroke (AIS), remains a significant problem in cardiac surgery, ranging from 1.5% to 5.2%. [13,16]. The development of stroke after CABG is accompanied by an increase of hospital mortality from 1-2% to 13-41%, increased length of hospital stay, and decreased quality of patient life [9].The pathogenesis of AIS development after CABG is multifactorial and includes embolic, hemodynamic, and inflammatory mechanisms [5]. The main risk factors for stroke development include age over 70 years, atherosclerosis of the ascending aorta and aortic arch, brachiocephalic artery stenosis, atrial fibrillation, diabetes mellitus, arterial hypertension, chronic heart failure, and previous cerebrovascular events [7,14].The relevance of the problem is associated with the severity and high disability of patients with AIS, as well as the lack of unified algorithms for prediction and prevention of this complication. The aim of the study was to develop a system for predicting the risk of acute ischemic stroke in patients with indications for coronary artery bypass grafting and to determine the optimal methods of prevention of this complication.
2. Material and Methods
- The study included 30 patients (22 men and 8 women) with development of acute ischemic stroke after coronary artery bypass grafting The mean age of the patients was 68.7±7.2 years. All patients had indications for CABG according to current clinical guidelines [15]. Exclusion criteria were as follows: emergency surgery, combined interventions (CABG + correction of valve defects, left ventricular aneurysms), history of AIS less than 6 months before surgery.All patients underwent a comprehensive preoperative examination, including:• Clinical and laboratory tests (general and biochemical blood tests, coagulogram, lipidogram, inflammation markers);• Instrumental studies: electrocardiography, echocardiography, coronary angiography;• Neuroimaging: computed tomography of the brain;• Ultrasound duplex scanning of the brachiocephalic arteries (USDS BCA);• Assessment of cognitive status using the MMSE scale (Mini-Mental State Examination).CABG surgeries were performed under artificial circulation using membrane oxygenators and nonpulsatile blood flow. The duration of artificial circulation was 98.2±22.5 min, the time of aortic clamping was 56.3±14.8 min. Intraoperative monitoring included invasive measurement of arterial pressure, central venous pressure, transesophageal echocardiography.The diagnosis of AIS was established on the basis of clinical picture and CT/MRI data of the brain. The NIHSS scale (National Institutes of Health Stroke Scale) was used to evaluate stroke severity.Single and multivariate logistic regression analysis was performed to identify risk factors for the development of AIS. Based on the obtained data, a prognostic model was developed and a risk index for the development of AIS was calculated. ROC analysis with calculation of area under the curve (AUC) was used to evaluate the informative power of the model.Statistical processing was performed using SPSS Statistics 25.0 program. Quantitative data are presented as mean ± standard deviation (M±SD) or median and interquartile range (Me [25%; 75%]), depending on the nature of the data distribution. Qualitative data are presented as absolute and relative frequencies. Differences were considered statistically significant at p<0.05.
3. Results
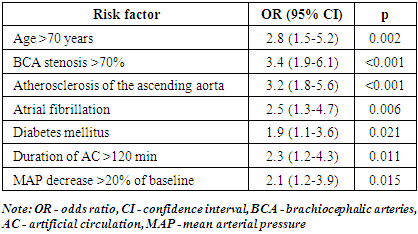
- In the studied 30 patients, AIS was developed on an average of 2.4±1.7 days after CABG surgery. By stroke localization: in the middle cerebral artery basin – 19 (63.3%) cases, in the vertebrobasilar basin – 8 (26.7%) cases, in the anterior cerebral artery basin – 3 (10%) cases. The stroke severity according to the NIHSS scale was 9.6±4.3 points.When analyzing the preoperative characteristics of patients with AIS, the following features were identified: Eighteen (60%) patients were over 70 years of age, 27 (90%) had arterial hypertension, 12 (40%) had diabetes mellitus, 9 (30%) had atrial fibrillation, 22 (73.3%) had chronic heart failure ≥II FC according to NYHA, and 23 (76.7%) had dyslipidemia. According to USDS BCA data, hemodynamically significant stenoses (>70%) of the brachiocephalic arteries were detected in 11 (36.7%) patients, atherosclerotic plaques in the ascending aorta (according to echocardiography and intraoperative revision) – in 15 (50%) patients.Multivariate logistic regression analysis identified independent predictors for the development of AIS after CABG (Tab. 1).
|
4. Discussion
- The results of the present study confirm the multifactorial nature of the development of AIS after CABG and are consistent with data obtained by other authors. Bucerius et al. [2] in a study involving over 16,000 patients also found that age >70 years, atrial fibrillation and diabetes mellitus were independent predictors of stroke after cardiac surgery.Atherosclerosis of the ascending aorta and aortic arch is a significant risk factor for the development of embolic stroke due to the mobilization of atheromatous masses during manipulations on the aorta (cannulation, clamping, formation of anastomoses) [6]. A meta-analysis by van der Linden et al. [17] showed that the use of the “no-touch aorta” technique or minimization of manipulations on the aorta can reduce the risk of neurological complications by 30-50%.Stenosis of the brachiocephalic arteries limits autoregulation of cerebral blood flow and increases the risk of developing both hemodynamic and embolic stroke. According to the recommendations of the European Society of Cardiology and the European Association of Cardiothoracic Surgeons [1], in the presence of significant stenosis of the internal carotid artery (>70%) and indications for CABG, preventive carotid endarterectomy or stenting should be considered.Artificial circulation time of more than 120 minutes increases the risk of systemic inflammatory response and microembolization, which in combination with hemostasis disorders may lead to the development of cerebral ischemia [4]. Interventions aimed at shortening AC time and the use of modern oxygenators with leukocyte filters help to reduce the incidence of neurologic complications.Intraoperative hypotension (decrease in mean arterial pressure by more than 20% of baseline) in conditions of impaired autoregulation of cerebral blood flow at brachiocephalic artery stenosis may be the cause of hemodynamic stroke [12]. Maintenance of adequate perfusion pressure and continuous monitoring of cerebral oxygenation by NIRS method allow to detect and correct cerebral hypoperfusion in time [10].The developed prognostic model with calculation of the AIS risk index after CABG demonstrated high informativity (AUC 0.85) and can be used to identify a group of high-risk patients requiring increased preventive measures. Similar risk stratification systems have been proposed by other authors, such as the NNECDSG scale [3] and the model of Charlesworth et al. [8], but they have limitations in the form of insufficient consideration of the state of brachiocephalic arteries and aorta.
5. Conclusions
- The main independent predictors of stroke development are age older than 70 years, brachiocephalic artery stenosis of more than 70%, atherosclerosis of the ascending aorta, atrial fibrillation, diabetes mellitus, prolonged artificial circulation, and intraoperative hypotension.A personalized approach to risk stratification and prevention of AIS at CABG can be integrated into clinical protocols for perioperative management of cardio surgical patients and contribute to improved outcomes of surgical treatment of coronary heart disease.
Conflict of Interests’ Statement
- The authors declare no conflict of interest. This study does not include the involvement of any budgetary, grant or other funds. The article is published for the first time and is part of a scientific work.
ACKNOWLEDGEMENTS
- The authors express their gratitude to the management of the multidisciplinary clinic of Republican Research Center of Emergency Medicine for the material provided for our study.
Ethical Approval and Consent to Participate
- The Research Ethics Board of our institution does not require review or approval of case reports. Our research was carried out in accordance with the World Medical Association Code of Ethics (Declaration of Helsinki).
Source of Funding
- Each of the authors has reviewed and approved this manuscript. None of the authors has a conflict of interest, financial or otherwise. This manuscript is original, no part of it has been previously published and is not being considered for publication elsewhere. The corresponding author agrees to accept full responsibility for authorship at the submission and review stages of the manuscript.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML