-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(6): 1855-1858
doi:10.5923/j.ajmms.20251506.46
Received: Apr. 12, 2025; Accepted: May 5, 2025; Published: Jun. 14, 2025

Analysis of Morphological and Morphometric Results of the Prostate Gland in 3-Month-Old White Outbred Rats with Experimentally Induced Pulmonary Fibrosis
Fayzullaev Komiljon Nabijonovich
Independent Researcher at Bukhara State Medical Institute, Uzbekistan
Correspondence to: Fayzullaev Komiljon Nabijonovich, Independent Researcher at Bukhara State Medical Institute, Uzbekistan.
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Introduction: This scientific study was conducted on white outbred rats of different ages with experimentally induced pulmonary fibrosis. In the study, bleomycin was administered endotracheally to 3-month-old white outbred rats, causing inflammation in the lungs and subsequently leading to pulmonary fibrosis. Methods: The dissertation research was conducted at the scientific research laboratory of Bukhara State Medical Institute from 2021 to 2024. For experimental studies, 86 white outbred male rats aged 3, 6, and 9 months were selected. The laboratory animals were kept in the vivarium of Bukhara State Medical Institute. In the scientific study, pulmonary inflammation and fibrosis were induced using the drug Bleomycin. Conclusion: Despite some anatomical and histological differences between human and white outbred rat prostate glands under normal conditions, numerous similarities were identified. The prostate gland of the control group of white outbred rats, anatomically distinct from the human prostate gland, consists of 4 separate lobes: anterior, lateral, ventral, and dorsal, based on the location of the excretory ducts in the urethra.
Keywords: Experimental pulmonary fibrosis, Pneumosclerosis, Experimental animals, Rat prostate gland, Morphological and morphometric examination
Cite this paper: Fayzullaev Komiljon Nabijonovich, Analysis of Morphological and Morphometric Results of the Prostate Gland in 3-Month-Old White Outbred Rats with Experimentally Induced Pulmonary Fibrosis, American Journal of Medicine and Medical Sciences, Vol. 15 No. 6, 2025, pp. 1855-1858. doi: 10.5923/j.ajmms.20251506.46.
Article Outline
1. Introduction
- Currently, idiopathic pulmonary fibrosis is understood as a chronic progressive fibrotic interstitial aseptic pneumonia of unknown etiology. Idiopathic pulmonary fibrosis (IPF) plays an important role in the spectrum of interstitial lung diseases. IPF is a fatal disease characterized by a progressive decline in quality of life, increasing limitation of physical functions, and premature death from respiratory failure. [1].The main virulence factor in the pathogenesis of COVID-19 is the interaction between the receptor-binding domain of the S protein, located on the outer membrane of the virus, and the angiotensin-converting enzyme 2 (ACE2) receptors. The development of COVID-19 is characterized by diffuse alveolar damage, which is determined by the formation of hyaline membranes, as well as the organization of alveolar exudate and interstitial fibrosis. [2]. The rate of inflammation and fibrosis development in the lungs is influenced not only by the volume and frequency of aspirations but also by the composition of aspirated materials. [3]. Thus, during the COVID-19 pandemic and due to various reasons, the development of idiopathic pulmonary fibrosis has been observed. [4]. Statistical data show that the reduction of pulmonary fibrosis and changes in the activity of other organs in the body as a result of pulmonary fibrosis are observed to varying degrees. Taking this into account, the prevention of pathologies arising from morphological changes occurring in the prostate gland tissue is one of the urgent problems of modern urology. [5].
2. Methodology
- The dissertation research was conducted at the scientific research laboratory of Bukhara State Medical Institute from 2021 to 2024. For experimental studies, 86 white outbred male rats aged 3, 6, and 9 months were selected. The laboratory animals were kept in the vivarium of Bukhara State Medical Institute. In the scientific study, pulmonary inflammation and fibrosis were induced using the drug Bleomycin. The following group of rats received Bleomycin endotracheally 4 times (1.5 IU/kg body weight) over a period of 1 month. The main objects of the study were histological preparations made from the prostate gland of white outbred male rats. Hematoxylin-eosin staining was used for general morphological examination. Among histochemical research methods, the VAN-Gieson method was used to identify collagen fibers specific to connective tissue in the tissue composition. In morphometric studies, data were obtained from morphometric indicators such as prostate mass (mg), epithelial surface area (μm2), stromal surface area (μm2), alveolar-tubular lumen surface area (μm2), epithelial height (μm), epithelial width (μm), and number of binucleated epithelial cells (units).
3. Results
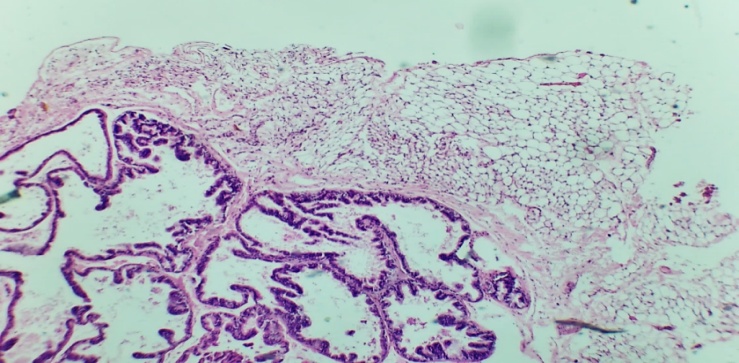
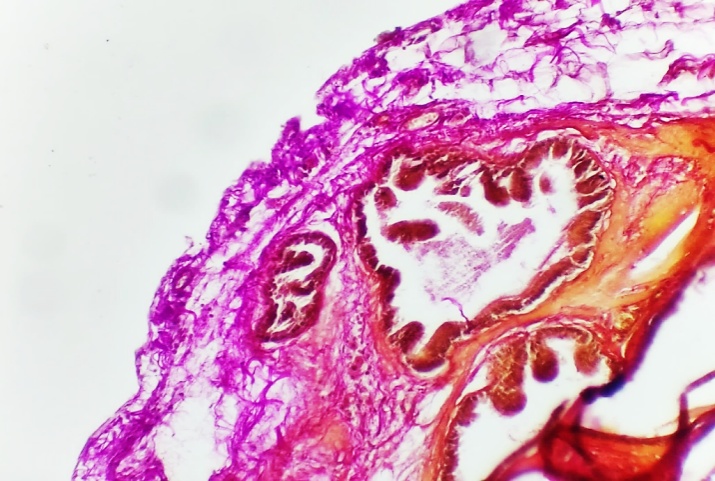
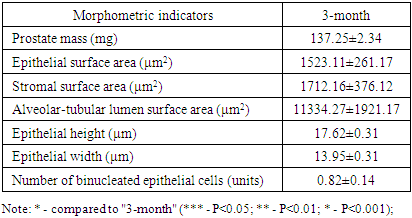
- The prostate gland anatomically differs among various laboratory animals. The prostate gland is usually located under the urinary bladder and in front of the rectum. There are many similarities in the histological structure of the prostate gland in laboratory animals and humans, but anatomically, in white outbred rats, the prostate gland consists of several lobes. The anatomical structure of the prostate gland in white outbred rats consists of four lobes, which have morphologically different aspects. These are the ventral lobe, lateral lobe, dorsal lobe, and anterior lobe. The naming of these lobes is based on their location relative to the bladder opening into the urethra. Despite the anatomical differences between human and white outbred rat prostate glands, the similarities in their histological, functional, and molecular mechanisms allow for experimental studies of various prostate disease models that occur in humans using these laboratory animals.The prostate gland of a white outbred rat is a tubular alveolar exocrine gland. It consists of four separate lobes: the dorsal prostate gland, the lateral prostate gland, the ventral prostate gland, and the anterior or coagulating gland, named after the location where their ducts open into the urethra. The above-mentioned parts have their own distinct histological features.Histologically, the lobules of the prostate gland are surrounded by a thin mesothelial connective tissue capsule. Each lobule consists of separate alveoli and acini, as well as numerous branching excretory ducts that empty into the urethra. Each acinus in the prostate contains thin, sparse connective tissue cells, smooth muscle cells, blood vessels, nerve fibers, nerve ganglia, macrophages, and mast cells. Additionally, secretory cells and basal cells are located in the acini and their excretory ducts. Basal cells do not produce secretions, and besides these cells, neuroendocrine cells are also present. The shape of secretory cells varies from cuboidal to columnar, depending on their secretory activity. The secretory activity and elasticity of the gland cause changes in the height of the cells in the acini. The acini are surrounded by smooth muscle fibers, and the complex contraction of these muscles facilitates the release of prostate gland secretions from the acini into the urethra through the excretory ducts.
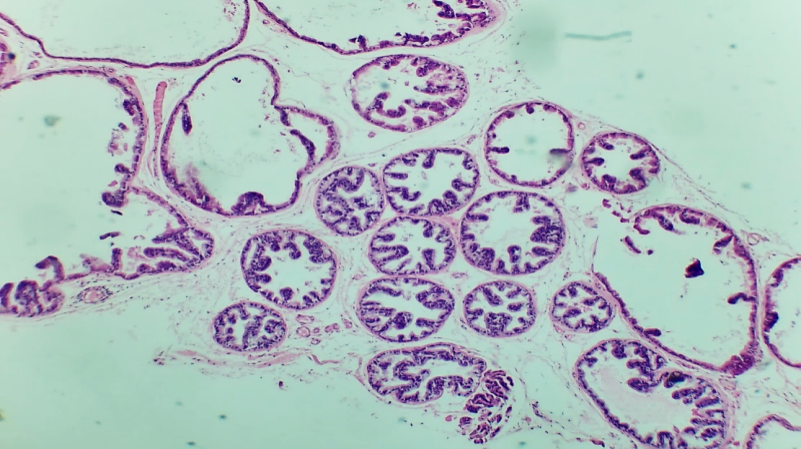 | Figures 1. Prostate gland of a 3-month-old white outbred |
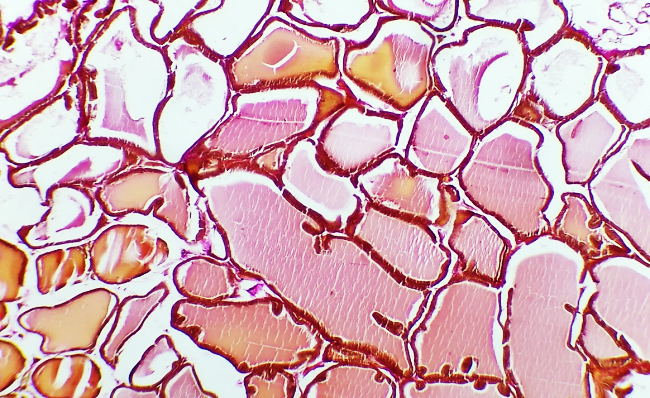 | Figures 2. Fine collagen fibers in the basal layer and stroma of the prostate gland |
|
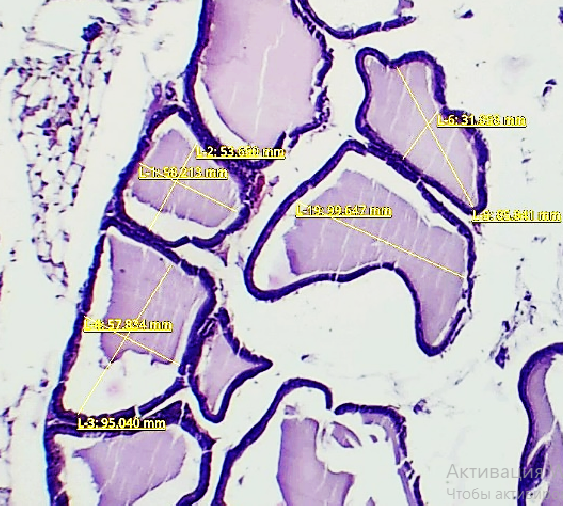 | Figure 3. Shows the morphometric measurements of the prostate gland in 3-month-old white outbred rats from the control group |
|
4. Conclusions
- Despite some anatomical and histological differences between human and white outbred rat prostate glands under normal conditions, numerous similarities were identified. The prostate gland of the control group of white outbred rats, anatomically distinct from the human prostate gland, consists of 4 separate lobes: anterior, lateral, ventral, and dorsal, based on the location of the excretory ducts in the urethra. Although there are some histological differences in the structure of these lobes, the similarity in their histological structure, molecular mechanisms, and functional properties allows for the study of prostate diseases in experiments using these laboratory animals.Macroscopically, an increase in the amount of fatty tissue surrounding the prostate gland, thickening and opacity of the capsule surrounding the gland, and signs of congestion in the blood vessels located under the capsule were observed. Microscopically, the prostate gland capsule was unevenly thickened, with congestion in the blood vessels, especially in the veins. The vessel walls were thickened, and signs of intravascular stasis were noted. An increase in interstitial edema, leukocyte cells, and collagen fibers was revealed in the gland stroma. In the glandular acini, flattening of the epithelial folds was observed, along with a decrease in the height of epithelial cells, which took on a cuboidal shape. There was a decrease in vesicles on their apical surfaces, as well as signs of congestion of glandular secretion in the acini and small concretions in some areas. In the experiment, it was established that in the group with induced pulmonary fibrosis, compared to the control group, the mass of the gland increased by 9.5% at 3 months, the surface area of the stroma increased by 6.5% at 3 months, and the surface area of the alveolar cavity increased after 3 months. It was found that the height of epithelial cells decreased by 2% at 3 months, and the width decreased by 0.5% at 3 months, while the surface area of epithelial cells in the tissue decreased by 4% at 3 months. The number of binucleated epithelial cells increased by 7% at 3 months. These morphometric indicators show that pulmonary fibrosis has a significant effect on the histological structures of the prostate gland.
Funding
- This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interests
- All the authors declare no conflict of interest.
CRediT Authorship Contribution
- The authors would like to thank the Bukhara State Medical Institute for supporting this study and providing the data through the Bukhara State Medical Institute Central Library.
Ethics Approval and Consent to Participate
- Not applicable.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML