-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(6): 1757-1766
doi:10.5923/j.ajmms.20251506.26
Received: Apr. 25, 2025; Accepted: May 28, 2025; Published: Jun. 7, 2025

Influence of Anesthetic Techniques on Oncological Outcomes in Surgery of Upper Abdominal Organs
Iskandarova Shakhnoza Tulkinovna1, Khakimova Laylo Nurali qizi1, Yusupov Anvar Sobirovich2
1Department of Public Health and Healthcare Management, Tashkent Pediatric Medical Institute, Tashkent, Uzbekistan
2Associate Professor of the Department of Anesthesiology and Resuscitation, Tashkent Pediatric Medical Institute, Tashkent, Uzbekistan
Correspondence to: Iskandarova Shakhnoza Tulkinovna, Department of Public Health and Healthcare Management, Tashkent Pediatric Medical Institute, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The influence of anesthetic techniques on cancer biology has emerged as a pivotal consideration in the perioperative management of patients undergoing surgery for upper abdominal malignancies. Increasing evidence indicates that volatile anesthetics and opioids may promote immunosuppression, angiogenesis, and tumor progression, while propofol-based total intravenous anesthesia (TIVA) and regional techniques demonstrate immunoprotective and anti-inflammatory properties. This review synthesizes mechanistic, clinical, and pharmacological insights, emphasizing the biological consequences of anesthetic choice in gastric, hepatic, pancreatic, and biliary cancer surgery. The role of the anesthesiologist is redefined as an active contributor to oncological outcomes, with implications for personalized medicine and integrated cancer care.
Keywords: Anesthetic technique, Cancer recurrence, Upper abdominal malignancies, Immunomodulation, Propofol, Volatile agents, Onco-anesthesia
Cite this paper: Iskandarova Shakhnoza Tulkinovna, Khakimova Laylo Nurali qizi, Yusupov Anvar Sobirovich, Influence of Anesthetic Techniques on Oncological Outcomes in Surgery of Upper Abdominal Organs, American Journal of Medicine and Medical Sciences, Vol. 15 No. 6, 2025, pp. 1757-1766. doi: 10.5923/j.ajmms.20251506.26.
Article Outline
1. Introduction
- The trajectory of oncological outcomes has traditionally been attributed to intrinsic tumor biology, molecular characteristics, and the radicality of surgical intervention. However, in the past two decades, a new axis of influence has emerged at the intersection of anesthesiology and oncology — one that challenges the conventional neutrality of anesthetic management and positions it as a potential modulator of tumor behavior and metastatic evolution [1], [2], [3]. This paradigm shift is grounded in growing evidence that perioperative interventions, particularly anesthetic techniques and agents, may exert effects far beyond the immediate operative period.Surgeries involving upper abdominal malignancies — namely, gastric, pancreatic, hepatic, and biliary tumors — represent some of the most immunologically destabilizing procedures in surgical oncology. These operations elicit a robust neuroendocrine and cytokine response, disrupt mucosal and endothelial integrity, and provoke a transient, yet profound, suppression of both innate and adaptive immune functions [4], [5], [6]. The perioperative window, once considered an operational interval, is now increasingly viewed as a biologically vulnerable phase during which minimal residual disease may either be eliminated by an intact immune system or, conversely, facilitated in its escape, seeding, and proliferation under the influence of surgical and anesthetic stress.Several mechanistic hypotheses support this re-evaluation. Volatile anesthetics, such as sevoflurane and desflurane, have been shown to activate hypoxia-inducible pathways (e.g., HIF-1α), upregulate vascular endothelial growth factor (VEGF), and promote epithelial-mesenchymal transition — all of which are implicated in tumor invasiveness and angiogenesis [7], [8], [9]. Moreover, volatile agents may impair natural killer (NK) cell cytotoxicity and alter cytokine profiles in a direction favorable to tumor escape. In contrast, intravenous agents like propofol exhibit anti-inflammatory, anti-oxidative, and anti-proliferative effects. Propofol has demonstrated the ability to preserve cellular immunity, reduce circulating pro-inflammatory mediators, and inhibit the motility and adhesion of various cancer cell lines [10], [11].Beyond the pharmacological profile of anesthetics, the technique of anesthesia itself — including the use of regional blocks, systemic lidocaine infusions, and opioid-sparing protocols — may influence perioperative immunocompetence and, consequently, the long-term trajectory of oncological disease [12], [13]. Yet, despite promising experimental and observational data, the clinical literature remains inconsistent. Many studies are limited by methodological heterogeneity, retrospective design, and insufficient stratification by tumor biology and perioperative variables [14], [15], [16].Given the increasing survival expectations and complexity of multimodal treatment strategies in oncological patients, the anesthesiologist is no longer a neutral participant but a co-architect of the biological field in which oncological fate is partially determined. The growing discipline of onco-anesthesiology demands a critical rethinking of perioperative management not only as a technical necessity but as a biological intervention with therapeutic consequence.This review aims to synthesize current knowledge regarding the impact of anesthetic techniques on oncological outcomes in the context of upper abdominal surgery. Special attention is devoted to the pathophysiological mechanisms underlying this interaction, the pharmacodynamic properties of various anesthetic agents, the comparative analysis of anesthetic approaches, and the methodological challenges in the current evidence base. The review also explores future perspectives and outlines a clinical framework for integrating anesthetic strategy into the broader continuum of oncologic care.
2. Pathophysiological Mechanisms Linking Anesthesia to Cancer Progression
- The notion that anesthetic management during oncological surgery may transcend its traditional supportive role and exert a measurable impact on long-term tumor behavior has transformed the conceptual boundaries of perioperative medicine. Today, there is a growing consensus that anesthetics can influence the trajectory of malignant disease through a range of immunological, molecular, and microenvironmental mechanisms — particularly relevant in surgeries of the upper abdominal organs, where systemic inflammation, immunosuppression, and tissue hypoxia are often pronounced [1], [3], [7].
2.1. Perioperative Immunosuppression and Impaired Antitumor Surveillance
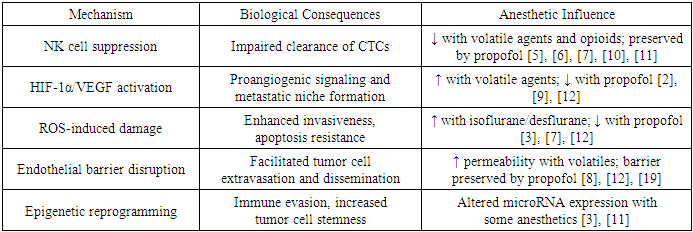
- One of the most studied and clinically relevant effects of anesthesia in the oncological setting is its capacity to suppress components of the innate immune system, most notably natural killer (NK) cells. These cytotoxic lymphocytes serve as the first line of defense against circulating tumor cells (CTCs), particularly during the perioperative period when hematogenous dissemination is most likely [5], [6]. Volatile anesthetics such as sevoflurane and halogenated ethers, as well as systemic opioids, have been associated with reduced NK cell activity, diminished interferon-γ production, and impaired dendritic cell function [7], [8]. In contrast, propofol has demonstrated an ability to preserve immune competence and may partially counterbalance the immunosuppressive effects of surgical trauma [10], [11], [17], [18].
2.2. Angiogenesis, Hypoxia Signaling, and Tumor Neo-Vascularization
- Another key mechanism through which anesthetic agents may alter tumor biology is the modulation of hypoxia-inducible factor 1-alpha (HIF-1α) and vascular endothelial growth factor (VEGF) expression. Volatile anesthetics have been shown to promote HIF-1α activation and stimulate VEGF-mediated angiogenesis, which supports the viability and expansion of micrometastases, particularly in hypoxic and nutrient-depleted environments typical of the postoperative state [2], [9]. Conversely, experimental studies have demonstrated that propofol may inhibit these proangiogenic pathways and suppress vascular remodeling, offering a potentially protective effect against metastatic progression [7], [12].
2.3. Oxidative Stress and Mitochondrial Instability
- Surgical trauma and anesthetic exposure also provoke the generation of reactive oxygen species (ROS), which induce DNA fragmentation, alter mitochondrial permeability, and activate intracellular signaling cascades linked to tumor cell motility and resistance to apoptosis [7], [12]. Volatile anesthetics, particularly isoflurane and desflurane, exacerbate oxidative injury and disturb redox homeostasis. In contrast, propofol, due to its phenolic structure, exhibits intrinsic antioxidant properties, stabilizes mitochondrial membranes, and may thereby limit ROS-induced oncogenic signaling [3], [10].
2.4. Endothelial Dysfunction and Facilitation of Metastatic Dissemination
- An often-overlooked factor in perioperative metastasis is the vascular endothelial barrier, which regulates the transmigration of CTCs into distant tissues. Anesthetics have been shown to alter endothelial junction integrity and vascular permeability. Volatile agents may promote nitric oxide–mediated vasodilation and destabilize endothelial adhesion molecules, facilitating extravasation of tumor cells into the circulation [8], [19]. By contrast, propofol has demonstrated the capacity to maintain endothelial structure and suppress cytokine-induced barrier dysfunction, potentially mitigating metastatic risk [12].
2.5. Epigenetic Modulation and Transcriptional Reprogramming
- Beyond immediate biochemical alterations, anesthetics may induce long-lasting epigenetic changes that alter tumor and immune cell behavior. This includes modulation of microRNA expression, histone acetylation, and DNA methylation patterns that influence cellular differentiation, immune evasion, and tumor cell stemness [3], [11]. While clinical translation of these findings remains in early stages, the evidence suggests that the epigenomic landscape of the tumor microenvironment may be susceptible to perioperative modulation, with implications for disease recurrence and therapeutic resistance.The mechanisms outlined above suggest that anesthesia in oncologic surgery is not merely a pharmacological necessity but a biological intervention with systemic consequences. The interplay between anesthetic agents, surgical trauma, and the host immune system may tip the balance between tumor dormancy and reactivation. A summary of these mechanistic pathways is presented in Table 1, which consolidates current knowledge regarding the pathophysiological influence of anesthetics on tumor progression.
|
3. Comparative Analysis of Anesthetic Techniques in Upper Abdominal Cancer Surgery
- The anesthetic approach in oncological surgery of upper abdominal organs — including gastrectomy, pancreaticoduodenectomy, and hepatic resection — is shaped not only by intraoperative stability and recovery metrics but increasingly by its potential long-term impact on tumor biology. The choice between volatile, intravenous, and regional anesthetic modalities may influence residual tumor cell viability, postoperative immune competence, and ultimately oncological outcomes [1], [4], [7].
3.1. Volatile Anesthesia: Biological Costs of a Conventional Standard
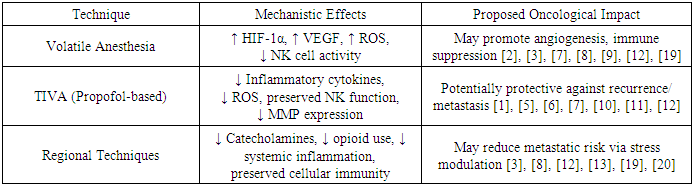
- The Volatile anesthetics such as sevoflurane, isoflurane, and desflurane remain widely used in major oncologic procedures due to their controllability and ease of titration. However, numerous in vitro and in vivo studies have raised concerns regarding their pro-tumorigenic potential. These agents have been shown to activate hypoxia-inducible factor pathways, increase VEGF expression, and promote matrix metalloproteinase activity, all of which contribute to tumor angiogenesis, epithelial–mesenchymal transition, and metastatic dissemination [2], [3], [7], [9].Moreover, volatile agents are implicated in immunosuppressive modulation, reducing natural killer (NK) cell cytotoxicity and altering cytokine profiles in favor of tumor tolerance. The oxidative stress induced by these agents may further disturb redox-sensitive signaling cascades within the tumor microenvironment [8], [12], [19].
3.2. Total Intravenous Anesthesia (TIVA): Propofol and the Protective Hypothesis
- Total intravenous anesthesia (TIVA), primarily using propofol, has attracted attention due to its anti-inflammatory, antioxidant, and anti-proliferative properties. Propofol has been shown to inhibit HIF-1α and VEGF expression, preserve endothelial integrity, and downregulate pro-metastatic mediators such as matrix metalloproteinases and adhesion molecules [5], [7], [10].Clinically, retrospective analyses have demonstrated lower recurrence rates and improved disease-free survival in patients receiving TIVA during cancer surgery, including those with upper gastrointestinal and hepatobiliary malignancies [6], [11], [12]. Additionally, propofol appears to sustain perioperative immune function more effectively than volatile agents, preserving NK cell activity and promoting anti-tumor cytokine responses [1], [10].
3.3. Regional Anesthesia: Immunomodulatory and Opioid-Sparing Effects
- Regional techniques — including epidural anesthesia, paravertebral blocks, and transversus abdominis plane (TAP) blocks — are increasingly integrated into multimodal oncologic anesthesia protocols. These techniques not only provide superior analgesia but may also reduce perioperative opioid consumption, thereby mitigating opioid-induced immunosuppression [3], [8], [20].Furthermore, regional blockade may blunt the neuroendocrine stress response, reducing catecholamine surge and systemic inflammation — both of which are implicated in metastatic activation. While data remain limited and heterogeneous, early clinical trials suggest a protective immunological profile when regional techniques are used in combination with TIVA [12], [13], [19].A summary comparison of the major anesthetic approaches and their proposed oncological effects is presented in Table 2, emphasizing their respective influence on angiogenesis, immune modulation, and tumor cell behavior.
|
4. Pharmacological Focus: Influence of Specific Agents
- Modern anesthetic pharmacology has evolved into a discipline deeply intertwined with immuno-oncology, tumor biology, and perioperative molecular signaling. Increasing evidence suggests that various agents commonly used in oncological anesthesia — including intravenous hypnotics, volatile anesthetics, opioids, and adjuncts — can directly or indirectly influence cancer outcomes by modulating immune function, inflammatory pathways, angiogenesis, and even epigenetic programming [7], [21], [22].
4.1. Propofol: From Sedation to Molecular Modulation
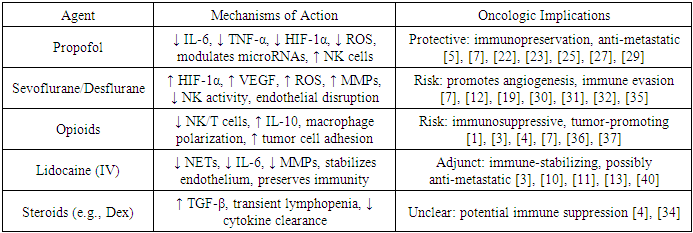
- Propofol, the mainstay of total intravenous anesthesia (TIVA), exerts numerous antitumor effects through multiple mechanisms. These include suppression of pro-inflammatory cytokines (IL-6, TNF-α), downregulation of HIF-1α and VEGF, inhibition of oxidative stress, stabilization of endothelial function, and protection of mitochondrial integrity [5], [7], [23]. Propofol also influences the IL-6/JAK/STAT3 signaling axis, which is implicated in cancer progression, particularly in gastrointestinal malignancies [22], [24].Furthermore, studies have shown that propofol alters microRNA expression within extracellular vesicles, thereby modulating tumor-related gene networks [7]. Multiple retrospective and meta-analytic studies have linked propofol-based anesthesia to improved recurrence-free survival and overall outcomes in colorectal, pancreatic, hepatic, breast, and ovarian cancer surgeries [25], [26], [27], [28], [29].
4.2. Volatile Anesthetics: Efficiency vs. Oncologic Safety
- Volatile agents such as sevoflurane, desflurane, and isoflurane are widely used due to their rapid onset and titration capability. However, several experimental and clinical studies have raised concerns about their proangiogenic and immunosuppressive properties. These agents upregulate HIF-1α and VEGF, increase ROS and MMP production, and suppress NK cell activity [2], [7], [19], [30], [31].Sevoflurane, in particular, has been associated with microenvironmental hypoxia, endothelial barrier dysfunction, and increased migration of tumor cells via epigenetic and transcriptional pathways [12], [32], [33], [34]. Meta-analyses suggest a potential association between volatile anesthesia and higher cancer recurrence rates, although definitive conclusions remain elusive [30], [31], [35].
4.3. Opioids: Immunosuppressive Consequences and Alternatives
- Opioids remain essential in perioperative analgesia, yet their impact on the immune system raises concerns. High-dose opioids such as morphine and fentanyl reduce NK cell activity, impair T-cell responses, and promote IL-10 secretion, potentially facilitating tumor immune evasion [1], [3], [36]. Additionally, opioids may influence tumor microenvironment remodeling and angiogenesis through macrophage polarization and modulation of adhesion molecules [4], [7], [37].Recent literature promotes opioid-free anesthesia (OFA) and opioid-sparing techniques as promising approaches to minimize such risks [1], [38]. Regional techniques and adjuncts such as lidocaine and NSAIDs form the cornerstone of these strategies [6], [39].
4.4. Lidocaine: Systemic Anti-Inflammatory Potential
- Lidocaine, traditionally used as a local anesthetic, has shown systemic anti-inflammatory and anti-metastatic effects when administered intravenously. It reduces NET formation, downregulates IL-6 and MMPs, and stabilizes endothelial function [10], [12], [13]. In breast cancer surgery, lidocaine-based protocols were associated with favorable cytokine profiles and reduced angiogenic activity [11].Randomized trials have begun to evaluate lidocaine’s role within enhanced recovery and immune-preserving protocols, with promising early results in gastrointestinal surgeries [3], [14], [40].
4.5. Additional Agents: Corticosteroids, NMBs, Remimazolam
- Corticosteroids such as dexamethasone are routinely used for antiemesis but may transiently suppress perioperative immunity through glucocorticoid receptor pathways [34]. Neuromuscular blockers have been associated with dose-dependent cytokine responses and altered immune cell recruitment, although data are heterogeneous [4], [7]. Remimazolam, a newer sedative-hypnotic, shows stable hemodynamics in oncologic settings, but its immunological impact remains under investigation [41].A consolidated comparison of the agents described above, with their relevant biological pathways and implications for cancer progression, is provided in Table 3.
|
5. Anesthetic Considerations in Minimally Invasive and Robotic Surgery
- The rapid evolution of minimally invasive surgical techniques — particularly laparoscopic and robot-assisted procedures — has profoundly transformed the landscape of upper abdominal cancer surgery. These techniques offer undeniable short-term benefits, including reduced surgical trauma, lower postoperative pain, decreased length of hospital stay, and accelerated recovery. However, their implementation introduces distinct anesthetic challenges and opportunities that must be carefully considered in the oncological context [42], [43], [44].Unlike open procedures, laparoscopic and robotic surgeries are associated with pneumoperitoneum-induced physiological alterations, including elevated intra-abdominal pressure, CO₂ retention, and changes in cardiac preload and afterload. These alterations may impact tissue perfusion and oxygenation, which in turn influence perioperative immune function and tumor microenvironment stability [45], [46].Robot-assisted procedures further amplify anesthetic complexity due to prolonged operative times, steep Trendelenburg positioning, and reduced access to the airway. These factors necessitate advanced intraoperative monitoring and often favor total intravenous anesthesia (TIVA) for its superior hemodynamic control and reduced risk of postoperative cognitive dysfunction, particularly in elderly cancer patients [41], [42], [47].From an oncological perspective, minimally invasive approaches have been associated with attenuated systemic inflammatory response, reduced catecholamine surge, and less disruption of natural killer (NK) cell activity compared to open surgery [39], [48]. These favorable immunologic effects may enhance tumor surveillance and reduce perioperative risk of metastatic spread. Furthermore, several studies suggest that the combination of minimally invasive surgery with propofol-based TIVA could potentiate immune preservation and minimize the immunosuppressive effects of surgical stress [26], [27], [29].Nevertheless, it is important to recognize that pneumoperitoneum and hypercarbia may also exert context-dependent effects on cytokine release, microcirculation, and endothelial barrier integrity, particularly in patients with preexisting comorbidities or advanced tumors [45], [46].Clinical evidence from randomized controlled trials and meta-analyses supports the non-inferiority or superiority of laparoscopic and robotic approaches in terms of long-term oncological outcomes, including disease-free and overall survival, when compared with traditional open resections [43], [44], [46]. These results are especially evident in procedures for gastric and esophageal malignancies, where minimally invasive total gastrectomy or Ivor Lewis esophagectomy has shown promising data [44], [45].In this context, anesthesiologists must be fully integrated into the surgical planning process, tailoring their strategies to maximize hemodynamic stability, minimize immunosuppression, and facilitate rapid postoperative recovery without compromising long-term cancer control. The choice of anesthetic agent, ventilation parameters, patient positioning, and fluid management becomes critical in optimizing outcomes for patients undergoing minimally invasive cancer surgery.
6. Pharmacological Focus: Influence of Specific Agents
- Surgical excision of malignancies, particularly in the upper abdomen, initiates a profound systemic response encompassing inflammation, oxidative stress, neuroendocrine activation, and transient immunosuppression. This perioperative window is not merely a physiological interlude — it is a critical determinant of residual tumor behavior, metastatic seeding, and long-term oncological outcomes. The influence of anesthetic agents on this phase, therefore, extends far beyond sedation and pain relief — it enters the realm of immuno-oncology, molecular signaling, and microenvironmental modulation [7], [21], [22], [34].
6.1. Innate Immune Surveillance and NK Cells
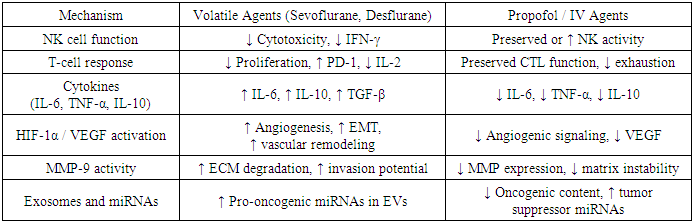
- Natural killer (NK) cells form the foundation of the innate antitumor defense, particularly crucial during surgical dissemination of circulating tumor cells (CTCs). Volatile anesthetics (e.g., sevoflurane, desflurane) and opioids have been shown to reduce NK cell cytotoxicity and suppress IFN-γ production, weakening early immunosurveillance [1], [3], [7], [36]. Propofol-based anesthesia, in contrast, preserves NK cell activity, supporting effective lysis of CTCs and suppressing metastatic potential [5], [10], [11], [25]. Recent studies also highlight lidocaine’s role in stabilizing NK cell–mediated responses [12], [13], [40].
6.2. Adaptive Immunity and T-Cell Dynamics
- Cytotoxic T lymphocytes (CTLs), particularly CD8⁺ cells, execute antigen-specific responses against tumor remnants post-resection. Surgical stress, combined with volatile anesthetics and corticosteroids, has been associated with T-cell anergy, reduction in IL-2 production, and overexpression of immune checkpoint molecules such as PD-1 [3], [22], [34]. Conversely, TIVA and regional techniques help maintain CTL functionality and may enhance tumor-specific immunologic memory [5], [38], [49].
6.3. Cytokine Networks and Inflammatory Pathways
- The perioperative cytokine storm influences both tissue regeneration and tumor progression. High intraoperative IL-6, IL-10, and TGF-β levels have been associated with increased VEGF secretion, tumor cell survival, and mesenchymal transition [12], [32], [37]. Propofol and intravenous lidocaine significantly downregulate these cytokines, promoting a more balanced immune response and inhibiting downstream pro-tumor signaling [10], [11], [27], [40].In contrast, volatile anesthetics promote prolonged proinflammatory activation, oxidative injury, and endothelial activation — all of which facilitate extravasation and implantation of CTCs [2], [7], [19], [31].
6.4. Molecular Pathways: HIF-1α, VEGF, MMPs
- Hypoxia-inducible factor-1α (HIF-1α), vascular endothelial growth factor (VEGF), and matrix metalloproteinases (MMPs) are pivotal in establishing the metastatic microenvironment. Volatile agents induce HIF-1α expression, enhancing angiogenesis and vascular remodeling [7], [23], [30]. This is further exacerbated by MMP-9–mediated degradation of the extracellular matrix, enabling tumor cell invasion [12], [32], [48].In contrast, propofol suppresses HIF-1α and VEGF transcription, reduces MMP-9 activity, and may limit epithelial–mesenchymal transition (EMT), thereby curbing metastatic potential [27], [29], [31].
6.5. Exosomes, microRNAs, and Epigenetic Crosstalk
- Emerging evidence underscores the importance of epigenetic mechanisms — including the regulation of microRNAs (miRNAs) and exosomal content — in shaping the perioperative tumor response. Propofol modulates miRNA expression in extracellular vesicles, attenuating oncogenic signaling and altering immune–tumor interactions [7], [22]. Conversely, sevoflurane has been linked to increased exosome release containing proangiogenic and proinflammatory miRNAs [32], [50].This epigenetic modulation represents a promising biomarker axis for anesthetic risk stratification in precision oncology.
6.6. Summary: Immuno-Molecular Impact of Anesthetics
- Cumulatively, anesthetic agents influence a web of molecular and cellular pathways that determine the fate of residual tumor cells. The immunological, inflammatory, and epigenetic signatures induced during surgery may persist long after the anesthetic wears off. Therefore, the choice of anesthetic — including the specific agent, technique, and adjuncts — must be considered a modulator of cancer biology, not merely a facilitator of surgical conditions.A comprehensive synthesis of these mechanisms is presented in Table 4.
|
7. Pharmacological Focus: Influence of Specific Agents
- The accumulated body of experimental and clinical evidence unequivocally demonstrates that anesthetic techniques are not merely auxiliary components of cancer surgery, but rather biologically active interventions that may influence disease trajectory. This paradigm necessitates a redefinition of anesthesiology’s role — from physiological management to active participation in oncological strategy development.
7.1. Practical Imperatives for Perioperative Teams
- In upper abdominal oncologic procedures, the selection of anesthetic agents must reflect not only intraoperative safety and recovery metrics but also long-term consequences for immune surveillance, metastatic potential, and tumor dormancy. Volatile agents, particularly sevoflurane and desflurane, have been associated with the upregulation of hypoxia-responsive genes, impaired NK and T-cell function, and facilitation of microvascular tumor cell migration [7], [30], [31].In contrast, intravenous agents such as propofol exhibit a more favorable biological profile, demonstrating suppression of IL-6 and HIF-1α, attenuation of MMP-9 activity, and preservation of anti-tumor immunity [5], [10], [27]. The strategic inclusion of regional techniques, lidocaine infusions, and multimodal opioid-sparing approaches should therefore be considered not merely analgesic choices, but oncologically informed decisions [3], [11], [12], [38], [40].This knowledge mandates the integration of anesthesiologists into interdisciplinary tumor boards, ensuring that perioperative plans align with the broader goals of disease control and survivorship.
7.2. Toward Individualized Anesthetic Oncology
- The heterogeneity of tumor biology — encompassing genetic instability, immune phenotypes, and microenvironmental variability — presents an opportunity for precision anesthetic medicine. Emerging technologies now permit the preoperative profiling of circulating exosomes, miRNA signatures, cytokine panels, and angiogenic markers, enabling risk-adaptive anesthetic protocols [7], [22], [32], [48].In this context, a patient with a VEGF-overexpressing pancreatic carcinoma, for instance, may benefit from a protocol that minimizes volatile exposure and favors agents with anti-angiogenic properties. Such molecularly guided anesthetic planning may become an essential component of multimodal oncological care.
7.3. Evidence Gaps and the Need for High-Quality Trials
- Despite compelling mechanistic and retrospective data, definitive clinical guidance remains hampered by the paucity of prospective, multicenter randomized controlled trials (RCTs). Heterogeneity in anesthetic regimens, tumor types, and immune endpoints confounds interpretation and limits guideline development [19], [31], [51], [52].To establish evidence-based standards, future RCTs must incorporate tumor biology stratification, biomarker endpoints, and standardized perioperative immune monitoring. These trials will not only clarify the oncologic impact of anesthetic agents but also pave the way for regulatory integration of anesthetic choices into formal cancer treatment algorithms.
7.4. The Evolving Role of the Anesthesiologist in Oncology
- The contemporary anesthesiologist is no longer confined to hemodynamic stability and pain control. As perioperative biology emerges as a modifiable determinant of recurrence, the anesthesiologist must assume the role of translational specialist bridging surgical oncology and molecular immunology [1], [49], [50], [53], [54].Their contribution encompasses the selection of agents with immunologic neutrality or benefit, minimization of iatrogenic immunosuppression, and enhancement of perioperative resilience. In this expanded capacity, the anesthesiologist becomes a co-architect of oncologic outcomes, whose expertise may influence not only immediate safety but also long-term survival.
8. Conclusions
- Anesthesia in oncologic surgery has moved beyond the boundaries of procedural support to become a biologically active component of cancer care. Accumulating evidence demonstrates that anesthetic techniques and agents influence key mechanisms of tumor progression, immune modulation, angiogenesis, and epigenetic signaling [7], [12], [22], [27].Volatile anesthetics and high-dose opioids, though long considered standard, are now associated with pro-metastatic effects, suppression of NK and T-cell function, and the promotion of angiogenic and inflammatory pathways [1], [7], [30], [31]. In contrast, propofol-based TIVA, lidocaine infusion, and regional anesthesia techniques have shown consistent immunoprotective, anti-inflammatory, and potentially anti-tumoral properties [3], [5], [10], [11], [40].The current landscape necessitates a redefinition of the anesthesiologist's role — from intraoperative technician to an active partner in oncologic strategy. Their decisions have lasting implications on recurrence, survival, and the biological destiny of the tumor [50], [54].To transform these insights into clinical reality, there is a pressing need for large-scale, multicenter randomized trials with long-term oncologic endpoints. Moreover, the future lies in individualized anesthetic protocols, informed by the molecular and immunological profiles of both patient and tumor [22], [32], [48].In the era of precision medicine, anesthetic planning must be as personalized and evidence-based as chemotherapy or surgical technique. Only through such integration can anesthesia fulfill its emerging role as a therapeutic tool in cancer control.
ACKNOWLEDGEMENTS
- The authors express their sincere appreciation to the Department of Public Health and Healthcare Management and the Department of Anesthesiology and Resuscitation at the Tashkent Pediatric Medical Institute for their support and contributions to the development of this manuscript.
DISCLOSURE
- The authors declare that they have no conflicts of interest relevant to the content of this article. No funding sources influenced the preparation of this review.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML