-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(6): 1752-1756
doi:10.5923/j.ajmms.20251506.25
Received: Apr. 24, 2025; Accepted: Jun. 2, 2025; Published: Jun. 7, 2025

Inflammatory and Renal Biomarkers in Patients with Type 2 Diabetes Mellitus and Chronic Kidney Disease: A Comparative Study in the Southern Aral Sea Region
Najmutdinova Dilorom Qamaritdinovna, Sultanov Sherzod Boxodirovich
Tashkent Medical Academy, Uzbekistan
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

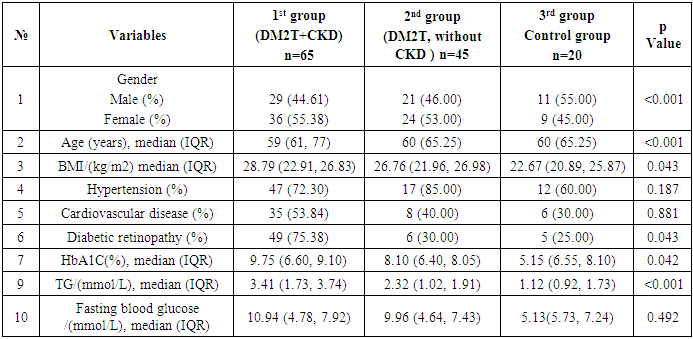
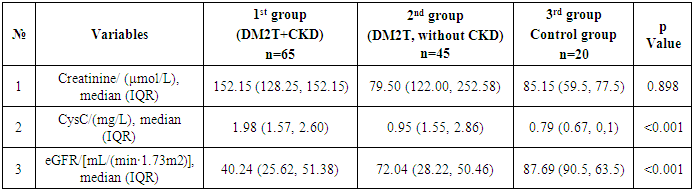
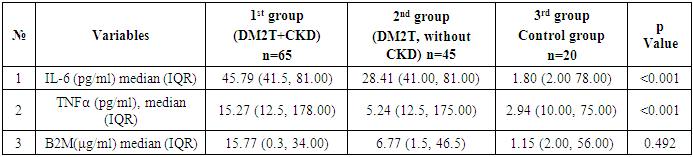
Introduction: Type 2 diabetes mellitus (T2DM) is a leading cause of chronic kidney disease (CKD), with inflammation and immune dysregulation playing key roles in disease progression. This study aimed to compare clinical, renal, and inflammatory markers in T2DM patients with and without CKD, and in healthy controls, in the environmentally stressed Southern Aral Sea region. Methods: A retrospective analysis was conducted on 130 individuals treated between December 2021 and December 2024. Participants were grouped as follows: T2DM with CKD (n=65), T2DM without CKD (n=45), and healthy controls (n=20). Clinical parameters, renal function (eGFR, cystatin C, creatinine), and inflammatory markers (IL-6, TNF-α, β2-microglobulin) were assessed. CKD was diagnosed using the CKD-EPI equation and urinary microalbuminuria according to NKF-KDOQI guidelines. Results: Patients with T2DM and CKD had significantly higher levels of IL-6 (median 45.79 pg/mL) and TNF-α (median 30.21 pg/mL) compared to other groups (p < 0.001), indicating increased systemic inflammation. Cystatin C levels were elevated (median 1.98 mg/L), and eGFR was significantly lower (median 40.24 mL/min/1.73m²) in the T2DM+CKD group (p < 0.001). No significant differences in creatinine or β2-microglobulin were observed between groups. HbA1c and triglyceride levels were also significantly higher in T2DM+CKD patients. Conclusions: Inflammatory and renal biomarkers are markedly altered in T2DM patients with CKD compared to those without CKD and healthy controls. These findings suggest that IL-6 and TNF-α may serve as important indicators of diabetic nephropathy progression. The results highlight the need for early identification of at-risk individuals, particularly in environmentally burdened regions such as the Southern Aral Sea.
Keywords: Diabetic nephropathy
Cite this paper: Najmutdinova Dilorom Qamaritdinovna, Sultanov Sherzod Boxodirovich, Inflammatory and Renal Biomarkers in Patients with Type 2 Diabetes Mellitus and Chronic Kidney Disease: A Comparative Study in the Southern Aral Sea Region, American Journal of Medicine and Medical Sciences, Vol. 15 No. 6, 2025, pp. 1752-1756. doi: 10.5923/j.ajmms.20251506.25.
1. Introduction
- Type 2 diabetes mellitus (T2DM) is a major global health concern and a leading cause of chronic kidney disease (CKD), which significantly increases the risk of cardiovascular morbidity and mortality. Diabetic nephropathy, a microvascular complication of T2DM, is characterized not only by progressive renal dysfunction but also by systemic inflammation and immune activation. In recent years, a growing body of evidence has emphasized the role of inflammatory mediators and non-traditional biomarkers in the pathogenesis and progression of diabetic kidney disease. Beta-2-microglobulin (B2M) is a small subunit of the major histocompatibility class I molecule, which is present on all nucleated cells [1]. Because it is non-covalently associated with the α chain and has no direct attachment to the cell membrane, free B2M circulates in blood after being shed from cell surfaces or by intracellular release. Once released, B2M is almost exclusively eliminated by glomerular filtration and has been used to determine the estimated glomerular filtration rate (eGFR). B2M concentration is fairly constant in healthy individuals [1], whereas blood levels of B2M increase in disease states such as renal dysfunction (due to reduced catabolism) and in certain malignancies, autoimmune diseases and infections (due to increased production). Serum B2M has been particularly useful as a clinical marker of chronic kidney-disease-related dysfunction [1].The association between higher B2M concentrations and mortality is well known for patients on maintenance dialysis therapy [2]. Serum B2M levels are greatly elevated in patients on dialysis and contribute to amyloid deposition, with associated cardiovascular dysfunction. Thus, serum B2M has been suggested to be a surrogate marker of cardiovascular disease in patients with chronic kidney disease (CKD) [2]. Although B2M is a marker of renal function, its effect on all-cause mortality was independent of renal function in a prospective study of 1034 non-disabled people aged ≥ 65 years [3]. Thus, serum B2M levels are a novel predictor of all-cause and diabetes-related mortality in patients with diabetes regardless of renal function [4]. B2M is susceptible to advanced glycation end-product (AGE) modification and glycation; the latter renders it cytotoxic [5]. However, we are unaware of any study that has investigated whether serum B2M is associated with diabetic complications in patients with type 2 diabetes (T2D). Therefore, the present study examined the association between serum B2M and diabetic complications (subclinical atherosclerosis and diabetic microvascular complications), and included only subjects with preserved kidney function to clarify the role of B2M independently of kidney function [2]. β2-microglobulin (β2-MG) is a microprotein formed by lymphocytes, polymorphonuclear leukocytes, and platelets, which has a positive effect on the inflammatory response [3]. Glycosylated hemoglobin (HbA1c) levels can reflect the specific control of blood glucose levels in patients in recent months. Excessively elevated HbA1c levels indicate the worsening of hyperglycemic injury in patients, increasing the influence of hyperglycemia on the development of microvascular lesions [4]. Considering all of these, this study examined the relationship between the expression of serum β2-MG, HbA1c, and the evaluation of DN patients, providing a scientific basis for clinical treatment and analysis of therapeutic effects.Among these biomarkers, beta-2 microglobulin (β2M), a low-molecular-weight protein filtered by the glomerulus and reabsorbed in the proximal tubule, has emerged as a potential marker of tubular dysfunction and early renal injury. Elevated β2M levels are associated with glomerular and tubular damage and may reflect ongoing immune activation in patients with T2DM and CKD.Pro-inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) are also increasingly recognized for their contributory roles in the development of diabetic nephropathy. IL-6 is involved in the acute phase response and promotes inflammation, while TNF-α mediates cellular injury and apoptosis, both of which are implicated in glomerular and tubular pathology. Elevated serum concentrations of these cytokines correlate with declining renal function and are thought to contribute to the chronic low-grade inflammation seen in T2DM.The Southern Aral Sea region represents a unique ecological and epidemiological setting, where environmental degradation, economic hardship, and limited healthcare access may exacerbate the burden of chronic diseases, including T2DM and CKD. Understanding the interplay between metabolic, inflammatory, and regional factors is essential for identifying at-risk populations and developing targeted interventions.This study aims to investigate the structural-functional and hemodynamic features of CKD in patients with T2DM residing in the Southern Aral Sea area, with a particular focus on the prognostic value of β2M, IL-6, and TNF-α as markers of renal and systemic inflammatory dysfunction.
2. Materials and Methods
- The study was planned according to ethical guidelines following the Declaration of Helsinki. This retrospective study evaluated the medical records of 130 adult patients, who were patients at the At the Nephrology Department of the Khorezm Regional Multidisciplinary Medical Center between December 2021 and December 2024. The patients were followed in the outpatient endocrinological clinic. Written informed consent for each participant was waived.Patients were diagnosed following the 1999 World Health Organization diabetes criteria and the estimated glomerular filtration rate (eGFR) and urinary microalbumin DKD criteria included in the NKF-KDOQI Guidelines [21,22]. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation was used to calculate the estimated glomerular filtration rate (eGFR) in mL/min/1.73 m2. The mean patient age was 59.27 ± 11.88 years of age, 66 were men and 64 were women. The cohort was divided into 1st group 65 (DM2T, CKD), and 2nd group 45 (DM2T, without CKD) and Control group patients. The pathogenesis and factors affecting the progression of сhronic kidney disease are different from those of type 2 diabetes, so they are not included in our study. Patient variables retrieved from their electronic medical records included sex, age, race, and medical history. Laboratory values included IL-6, β2-microglobulin, TNF-α, HbA1C, cholesterol, cystatin C, and eGFR, and other the specific lab values. Physiological data included DKD-associated complications and comorbid diseases including diabetic retinopathy, hypertension history, and cardiovascular disease.
3. Results
- We analyzed the concentration of total (inactive + active) TNF-α, IL-6, β2-microglobulin in DM2T patients complicated by CKD and without CKD in control subjects. Also, we studied a relation of TNF-α values with IL-6, B2M counts as well as with hematological and biochemical parameters in this patients. Finally, we tested if B2M and TNF-α values could be a prognostic factor in DM2T patients with DKD. The results are presented in Table 1 and Table 2.
|
|
|
4. Conclusions
- This study highlights the significant clinical, renal, and inflammatory differences among patients with type 2 diabetes mellitus (T2DM) with and without chronic kidney disease (CKD), as well as healthy controls in the Southern Aral Sea region. Patients with both T2DM and CKD exhibited higher levels of inflammatory markers such as IL-6 and TNF-α, alongside more pronounced renal impairment as indicated by elevated cystatin C and decreased eGFR values. The findings suggest that systemic inflammation, coupled with metabolic and hemodynamic dysregulation, may play a critical role in the progression of diabetic nephropathy.Furthermore, regional environmental and socioeconomic factors may contribute to the observed patterns, emphasizing the need for tailored preventive and therapeutic strategies in populations residing in ecologically vulnerable areas such as the Southern Aral Sea region. Early identification and monitoring of inflammatory and renal biomarkers could improve prognostication and management of T2DM-related complications, particularly CKD.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML