-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(6): 1732-1736
doi:10.5923/j.ajmms.20251506.21
Received: May 3, 2025; Accepted: May 23, 2025; Published: Jun. 5, 2025

Efficacy of Local Class IC Antiarrhythmic Drugs in the Early Post-Ablation Period Following Pulmonary Vein Isolation
Uralov Kh. I.1, Zakirov N. U.2, Amirkulov B. Dj.3
1PhD Student of Department of Cardiac Arrhythmias, Republican Specialized Cardiology Scientific Practical Medicine Center, Tashkent, Uzbekistan
2DSc, Professor of Department of Cardiac Arrhythmias of Republican Specialized Cardiology Scientific Practical Medicine Center, Tashkent, Uzbekistan
3DSc, Head of Department of Electrophysiological Study and Surgical Repair of Complex Types of Cardiac Arrhythmias of Republican Specialized Cardiology Scientific Practical Medicine Center, Tashkent, Uzbekistan
Correspondence to: Uralov Kh. I., PhD Student of Department of Cardiac Arrhythmias, Republican Specialized Cardiology Scientific Practical Medicine Center, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background. Although the efficacy of locally produced Class IC antiarrhythmic drugs (AAD) has been studied in various types of cardiac arrhythmias, their rhythm-maintaining efficacy during the blanking period (3 months) following pulmonary vein radiofrequency catheter ablation (RFCA) for atrial fibrillation (AF) has not been investigated to date. Objective. To compare the efficacy and safety of lappaconitine-based agents (aksaritmin/allapinin) versus propafenone during the blanking period following RFCA of pulmonary veins in patients with paroxysmal or persistent AF. Materials and methods. This prospective randomized controlled study included 54 patients consecutively undergoing RFCA for paroxysmal or persistent AF. All patients were divided into two groups: 1-group received lappaconitin (n=28), 2-group received propafenone (n=26) during 3 month. Outcomes included time to recurrence, rhythm maintenance time and adverse effects. Statistical analysis was performed using SPSS 27.0, with p<0.05 considered significant. Results. Baseline characteristics were comparable between groups. Early recurrence incidence rate 50% (1 group) and 46.2% (2 group) of patients. Mean of AF–free days 47.7±42 and 59.4±38.7 days, respectively (p=0.83). In the study group, arrhythmia recurrence manifested as atrial flutter (AFL) in 57.14% of cases and atrial fibrillation (AF) in 42.86%, whereas in the control group, AF predominated (83.3%) with AFL observed in only 16.7% of cases (p=0.035). AAD related adverse effects were not significantly different between groups. Conclusion. The locally produced Class IC antiarrhythmic drugs demonstrated non-inferiority to propafenone in maintaining sinus rhythm during the early post-ablation period following pulmonary vein catheter ablation. However, the higher incidence of atrial flutter observed in the intervention group suggests that the proarrhythmic potential of lappaconitine derivatives in relation to atrial flutter warrants further investigation.
Keywords: Atrial fibrillation, Pulmonary vein radiofrequency catheter ablation, Blanking period, Aksaritmin, Allapinin, Propafenone, Early recurrence, Atrial flutter
Cite this paper: Uralov Kh. I., Zakirov N. U., Amirkulov B. Dj., Efficacy of Local Class IC Antiarrhythmic Drugs in the Early Post-Ablation Period Following Pulmonary Vein Isolation, American Journal of Medicine and Medical Sciences, Vol. 15 No. 6, 2025, pp. 1732-1736. doi: 10.5923/j.ajmms.20251506.21.
Article Outline
1. Introduction
- Pulmonary vein isolation (PVI) is currently a widely used and effective treatment method for patients with symptomatic paroxysmal and persistent atrial fibrillation (AF). However, during the early post-ablation period — commonly referred to as the “blanking period” — the persistence of post-ablation thermal myocardial inflammation [1] and ongoing atrial electrical remodeling [2] may lead to the recurrence of atrial arrhythmias, including AF, atrial flutter (AFL), and other atrial tachyarrhythmias. These arrhythmias may necessitate cardioversion and hospital readmission [3].Maintaining sinus rhythm during this critical blanking period is essential for reducing the risk of long-term AF recurrence, preventing structural remodeling, and improving patients’ quality of life. For this purpose, Class IC antiarrhythmic drugs (AADs), particularly propafenone, are commonly used worldwide in patients without structural heart disease [4-5].In Uzbekistan, lappaconitine-based agents derived from Aconitum septentrionale, including aksaritmin and allapinin, have been studied for their efficacy and safety in managing both ventricular and atrial arrhythmias, and have shown promising rhythm-restoring effects comparable to propafenone. However, despite these findings, their prophylactic efficacy and safety during the blanking period following pulmonary vein catheter ablation for AF have not been investigated [6].Therefore, the aim of this study was to perform a comparative analysis of the efficacy and safety of lappaconitine-based antiarrhythmic agents versus propafenone during the blanking period after pulmonary vein catheter ablation.
2. Materials and Methods
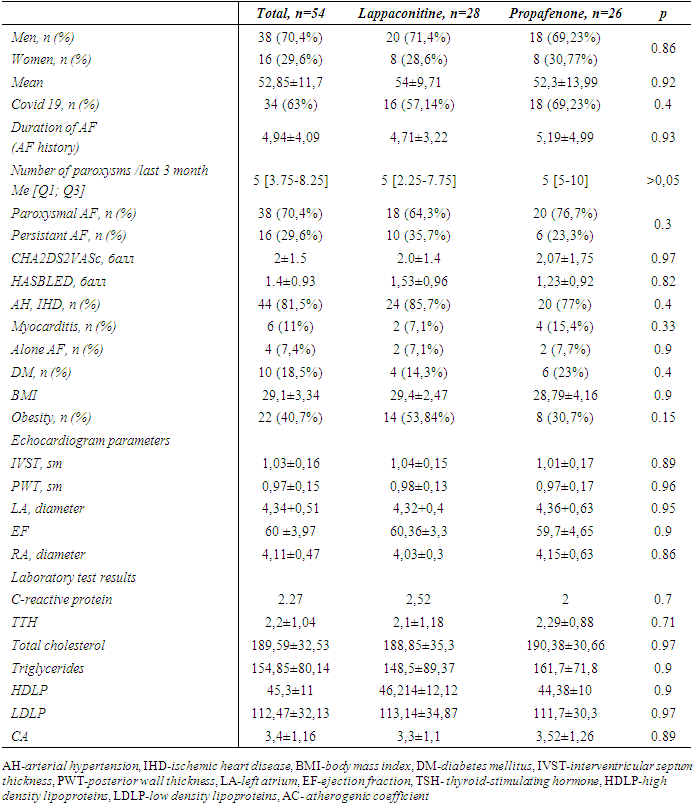
- This prospective, randomized, and open-label (non-blinded) clinical study enrolled male and female patients aged between 18 and 75 years who were referred to the Republican Specialized Cardiology Scientific and Practical Medical Center for first-time pulmonary vein radiofrequency ablation due to symptomatic and/or frequently recurring paroxysmal and persistent atrial fibrillation (AF) confirmed by ECG or Holter monitoring (>30sec). Inclusion criteria consisted of patients who had failed at least one antiarrhythmic drug.Exclusion Criteria: • Chronic atrial fibrillation (permanent); unwillingness to take the antiarrhythmic drugs (AADs) – propafenone and lapaconitine;• High sensitivity or inability to tolerate AADs;• New York Heart Association (NYHA) Class III and IV heart failure;• Structural heart diseases (mild or severe stenosis of the aortic and mitral valves, interventricular septum thickness ≥15 mm, congenital heart defects);• Pregnancy and lactation period;• Left atrial diameter (LAD) > 50 mm;• Hepatic and/or renal insufficiency;• Coagulopathy or bleeding disorders;• Severe infections, mental disorders, and malignancies;• Patient refusal to undergo catheter ablation;• Thyroid diseases.Informed Consent and Study Timeline: Written informed consent was obtained from all participants before their inclusion in the study. Patient enrollment in the study began in January 2023 and was completed in December 2024. The follow-up continued until April 2025.Radiofrequency catheter ablation (RFCA): Electrophysiological Study (EPS) was performed under fluoroscopic guidance (Siemens C-arm). Local anesthesia was applied, and puncture was performed in the right jugular vein and bilateral femoral veins. Under fluoroscopy, two PREFACE introducers (Biosense Webster) were advanced into the oval fossa area, through which a special needle for transseptal puncture was inserted into the interatrial septum. A left atrial angiography catheter was inserted, and the size and location of the pulmonary veins' ostial segments were determined. Subsequently, the angiography catheter was replaced with a variable diameter, steerable multi-channel Lasso electrode, and an electrode for ablation was introduced through the second introducer. Circular mapping of the pulmonary veins was performed through the Lasso catheter — the catheter was advanced from the ostial part of the vein to the distal sections, observing the loss of electrical activity. This allowed for the assessment of the size of the pulmonary vein muscle "sleeves." In sinus rhythm, two or more-component potentials were recorded around the ostial part of the pulmonary veins in all patients. The first — low-frequency potential — reflected the atrial wall activity, and the second — high-frequency potential — reflected the electrical activity of the pulmonary vein muscle sleeve. In cases where differentiating between the potentials of the left atrium and pulmonary veins was difficult, coronary sinus stimulation was performed, which allowed clear distinction. In cases of ectopic activity, reverse activation was recorded: first, a high-frequency venous potential, followed by a low-frequency potential near the ostium. Electrical isolation of the pulmonary veins' ostial segments was performed during sinus rhythm or coronary sinus stimulation, starting from the earliest activation point of the vein. The ostial part of the vein was divided into 12 sectors, with the goal of achieving complete electrical disconnection from the remaining part of the left atrium. For electrical isolation of the pulmonary veins' ostial segments, the Stockert ablation system (Biosense Webster) was used. Irrigated radiofrequency ablation was performed through irrigated catheters, with the following parameters: maximum temperature — 40–44°C, power — 30–35 W, physiological saline flow at the distal electrode — 17–30 mL/min. After ablation, the electrophysiological characteristics of all pulmonary veins were compared and evaluated: effective and functional refractory periods, ability to induce AF/AT in the isolated vein, fragmented activity, and conduction time.Antiarrhythmic treatment following RFCA: 54 patients who underwent consecutive pulmonary vein isolation (PVI) procedures were enrolled in the study. They were randomly assigned into two groups using a randomization method: 28 patients received lappaconitine-based antiarrhythmic therapy (Group 1), and 26 patients received propafenone (Group 2) for a duration of 3 months (blanking period). During the post-ablation period, all patients received standard therapy based on their underlying cardiovascular condition, along with anticoagulation therapy—administered for 3 months irrespective of the CHA₂DS₂-VASc score, and continued indefinitely in patients with a score of 2 or higher. Lappaconitine was administered (Aksaritmin, n = 22 or Allapinin, n = 6) at a dosage of 75–150 mg/day, while propafenone was prescribed at a dosage of 450-600 mg/day. All patients underwent electrocardiography (ECG), 24-hour Holter monitoring, and transthoracic echocardiography (TTE) at 1 and 3 months post-ablation. The main evaluation criteria were the duration of sinus rhythm maintenance, the occurrence of arrhythmic recurrences (atrial fibrillation or atrial flutter), and the incidence of adverse events (both cardiac and extra cardiac). In cases where clinical symptoms suggestive of AF/AFL (such as palpitations, chest discomfort, dyspnea, and/or sudden fatigue) were reported, patients were invited for evaluation. For those residing remotely from the electrophysiology center, ECG recordings were obtained locally and transmitted via telecommunication, followed by confirmation of rhythm disturbances (AF or AFL). In the event of arrhythmic recurrence on one antiarrhythmic drug (AAD), crossover to the alternative AAD was permitted. If recurrences persisted despite crossover therapy, amiodarone was initiated. In cases of atrial flutter, sinus rhythm was restored in an inpatient setting using intravenous amiodarone. Statistical analysis was conducted using SPSS version 27.0, with a p-value of <0.05 considered statistically significant. Baseline clinical characteristics of the patients at the time of PVI are presented in Table 1.
|
3. Results
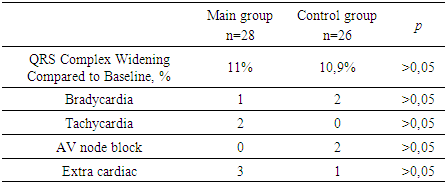
- No statistically significant differences in baseline patient characteristics were observed between the two groups. Early recurrences documented in 48% of patients during the blanking period. The rhythm control efficacy in Group 1 was 50% (14 patients), with a mean duration of sinus rhythm maintenance of 47.7 ± 42 days. In the propafenone group (Group 2), these figures were 53.8% (14 patients) and 59.4 ± 38.7 days, respectively [p = 0.83]. Among patients with recurrence in the main group, atrial flutter was observed in 57.14%, while atrial fibrillation occurred in 42.86%. In contrast, in the control group, atrial flutter was recorded in only 2 patients (16.7%), while atrial fibrillation was detected in 10 patients (83.3%) [p = 0.035].
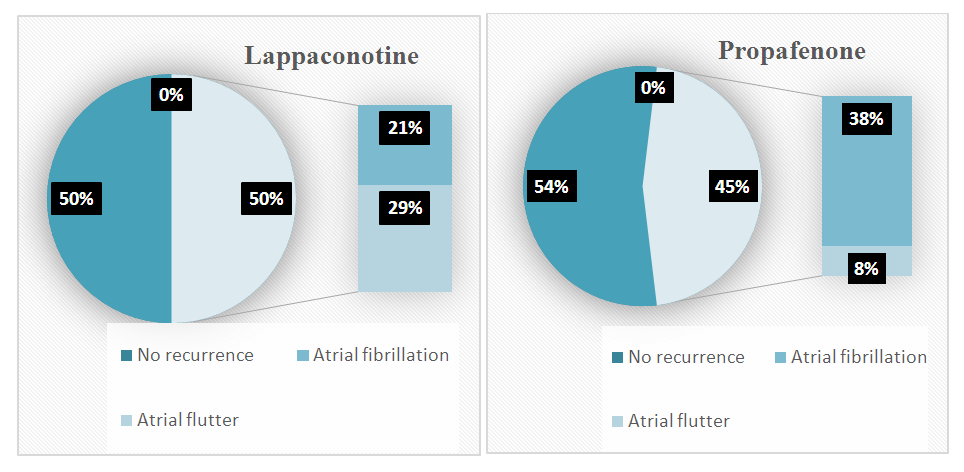 | Figure 1. Comparison of antiarrhythmic drug efficacy in maintaining sinus rhythm and types of recurrence during blanking period |
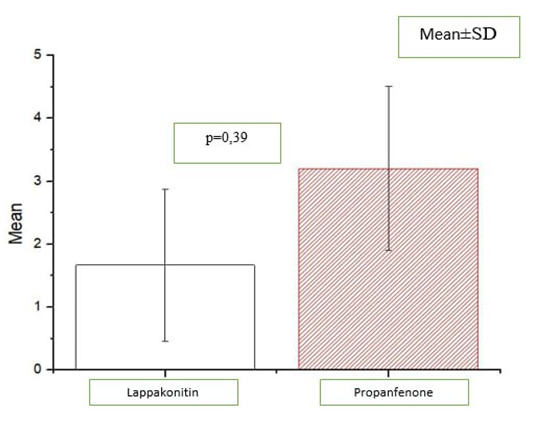 | Figure 2. Comparison of the number of arrhythmic episodes observed over 3 months between the groups |
|
4. Discussion and Conclusions
- In this study, the comparative analysis of the efficacy and safety of locally available antiarrhythmic drugs (Aksaritmin and Allapinin) versus the reference drug propafenone during the early period following pulmonary vein catheter ablation (PVCA) was conducted. The absence of significant differences in baseline clinical, anamnestic, and laboratory-instrumental parameters between the two groups ensured the reliability of the study outcomes.The findings showed that arrhythmia recurrences were observed in 48% of patients during the blanking period, which is consistent with data from international guidelines (16–67%) [1]. Nevertheless, the slightly lower recurrence rates in our study may be explained by the late referral of patients with paroxysmal atrial fibrillation (AF) for radiofrequency catheter ablation (RFCA), the relatively lower frequency of pulmonary vein isolation (PVI) procedures compared to other types of ablations (which could be influenced by the operator’s experience), and the fact that, prior to this study, PVI procedures were performed using conventional RF ablation without electroanatomic mapping systems.Although the blanking period is traditionally considered to last 3 months, several studies suggest the need for a re-evaluation of its optimal duration [7–9]. In our study, the number of AF-free days (47.7±42 vs. 59.4±38.7) suggests that post-ablation inflammatory processes, which may contribute to recurrences, can persist for longer than one month.The maintenance of sinus rhythm was achieved in 50% of patients receiving local antiarrhythmic drugs and in 53.8% of those treated with propafenone, with no statistically significant difference between the groups, supporting the non-inferiority of the local agents compared to the reference drug. However, differences were observed in the nature of the recurrences: atrial flutter was more frequently detected in the local antiarrhythmic group [57.14% vs. 16.7%, p=0.035], suggesting a possible role of these agents in promoting atrial flutter development after PVI, which requires further investigation.
Limitations
- The study was conducted on a relatively small number of patients, which may limit the generalizability of the findings to a broader population. Additionally, the small sample size made it difficult to separately assess the efficacy of treatment in patients with paroxysmal versus persistent AF. Furthermore, the lack of registration of implantable loop recorders in Uzbekistan led to reliance on subjective methods for detecting recurrences, potentially resulting in missed cases of asymptomatic AF episodes.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML