-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(6): 1725-1731
doi:10.5923/j.ajmms.20251506.20
Received: May 21, 2025; Accepted: Jun. 2, 2025; Published: Jun. 5, 2025

Relationship between Cytokines (TNF-α, IL-6, IL-10) and Procalcitonin in Elderly Uzbek’s with Type 2 Diabetes
Ergasheva Zilolakhon Nazarmat kizi1, Zalyalieva Mariyam Valiyevna2, Nuruzova Zukhra Abdukadirovna3
1PhD Candidate, Department of Microbiology, Virology and Immunology Tashkent Medical Academy, Tashkent, Uzbekistan
2MD, Dr. Sci., Professor Head of the Laboratory of Immune Physiology Institute of Immunology and Human Genomics Academy of Sciences of the Republic of Uzbekistan Tashkent, Uzbekistan
3MD, Dr. Sci., Professor, Head of the Department of Microbiology, Virology and Immunology, Tashkent Medical Academy, Tashkent, Uzbekistan
Correspondence to: Ergasheva Zilolakhon Nazarmat kizi, PhD Candidate, Department of Microbiology, Virology and Immunology Tashkent Medical Academy, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Currently, type 2 diabetes mellitus (T2DM) is a widespread disease among the elderly. The study included 60 patients with T2DM aged 60–74 years and a control group of 23 healthy native volunteers. Patients diagnosed with diabetic foot syndrome were excluded. Levels of pro-inflammatory (TNF-α, IL-6) and anti-inflammatory (IL-10) cytokines, as well as procalcitonin (PCT), were analyzed. Patients with T2DM showed a significant increase in TNF-α and IL-6 levels (by 3.8 and 9.2 times, respectively) compared to the control group. Women had higher PCT levels than men did (p<0.05). The integral ratio coefficient (K) of pro- and anti-inflammatory cytokines increased with rising blood sugar levels and body weight. A strong correlation was found between blood sugar levels and PCT (r=+0.90, p<0.001). Different degrees of association between PCT and cytokines were identified. The analysis of relationships between cytokine levels (TNF-α, IL-6, IL-10) and clinical indicators in elderly patients with T2DM revealed correlations between pro-inflammatory cytokines and HbA1c, as well as between IL-10 and PCT, emphasizing the role of inflammation in carbohydrate metabolism disorders. The findings confirm the importance of immunological changes in T2DM and their potential role in complications, which may be valuable for further studies on the disease pathogenesis.
Keywords: Type 2 diabetes mellitus, Cytokines, TNF-α, IL-6, IL-10, Procalcitonin, Elderly patients, Metabolic syndrome
Cite this paper: Ergasheva Zilolakhon Nazarmat kizi, Zalyalieva Mariyam Valiyevna, Nuruzova Zukhra Abdukadirovna, Relationship between Cytokines (TNF-α, IL-6, IL-10) and Procalcitonin in Elderly Uzbek’s with Type 2 Diabetes, American Journal of Medicine and Medical Sciences, Vol. 15 No. 6, 2025, pp. 1725-1731. doi: 10.5923/j.ajmms.20251506.20.
Article Outline
1. Introduction
- Currently, type 2 diabetes mellitus (T2DM) is a highly prevalent disease among the elderly. According to the International Diabetes Federation, the number of adults (aged 20–79 years) diagnosed with diabetes increased from 285 million in 2009 to 463 million in 2019, accounting for 95% of T2DM cases. If current trends persist, this number is expected to rise to an astounding 783 million (12.2%) by 2045. Among individuals aged 75–79 years, the highest diabetes prevalence was estimated at 24.0% in 2021, with projections suggesting an increase to 24.7% by 2045. A similar trend, with slight variations, is observed among elderly individuals aged 60–74 years [1-2].It is well known that elderly patients experience general age-related metabolic changes, which, in turn, influence the development of diabetes. Additionally, age-related impairments in the immune system, particularly a decline in T-cell immunity, are observed in patients with T2DM, leading to significant alterations in the cytokine network [3]. There have also been reports of changes in calcitonin metabolism in elderly COVID-19 patients [4]. However, our literature review did not find studies investigating the relationship between cytokine levels and procalcitonin (PCT) in elderly patients with T2DM.
2. Purpose of the Research
- To study the levels of cytokines (TNF-α, IL-6, IL-10) and PCT, their correlation with clinical parameters in elderly patients with T2DM, and their potential role in the mechanisms of chronic inflammation and glycemic control impairment.
3. Materials and Methods
- The study included 60 elderly patients aged 60–74 years diagnosed with type 2 diabetes mellitus (T2DM) according to medical records from the Ministry of Health of the Republic of Uzbekistan, approved by Order No. 363 on December 31, 2020 (Medical Document Form No. 003). The study population consisted of 33 men (55%) and 27 women (45%), all of whom were of Uzbek ethnicity. All patients were taking hypoglycemic drugs from the biguanide group.The patients had the following comorbid conditions: angina pectoris, chronic pyelonephritis, hypertension, anemia, and osteoarthritis, but without macroangiopathies (such as atherosclerosis of the lower limb vessels or diabetic foot syndrome). At the time of inclusion in the study, the mean duration of diabetes in the T2DM group was 11.8 ± 0.91 years. A comparison group of 23 healthy volunteers with BMI <30 kg/m² was selected and randomized by age and sex. The presence of metabolic syndrome was assessed according to the criteria of the International Diabetes Federation [5].Blood samples were collected from the cubital vein using vacutainers under aseptic conditions to evaluate all studied parameters. From the obtained serum, pro-inflammatory cytokines (TNF-α and IL-6), the anti-inflammatory cytokine (IL-10), and procalcitonin (PCT) were measured using ELISA, following the manufacturer’s recommendations and protocols [6-7], ensuring compliance with international quality standards.The collection of study material was carried out at the Multidisciplinary Clinic of the Tashkent Medical Academy, in the departments of endocrinology and purulent surgery, with voluntary informed consent from patients and approval from the clinic’s administration. The Institute of Immunology and Human Genomics of the Academy of Sciences of the Republic of Uzbekistan conducted the immunological study under an inter-institutional agreement.The collected data were analyzed using Microsoft Excel and GraphPad Prism 8.0.1 for visualization. An unpaired Student’s t-test was used to compare mean values between the two groups. Pearson’s correlation analysis was applied to determine correlations. A p-value <0.05 was considered statistically significant.
4. Results
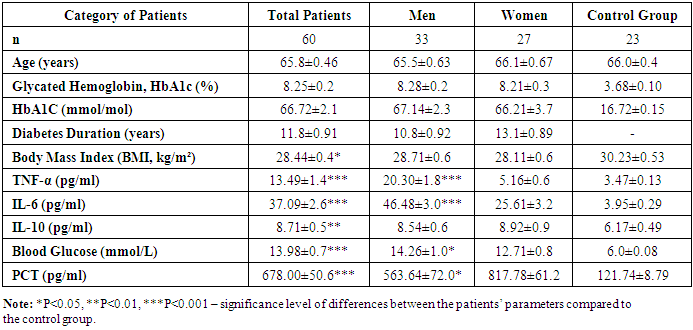
- The results of clinical and immunological studies are presented in Table 1.
|
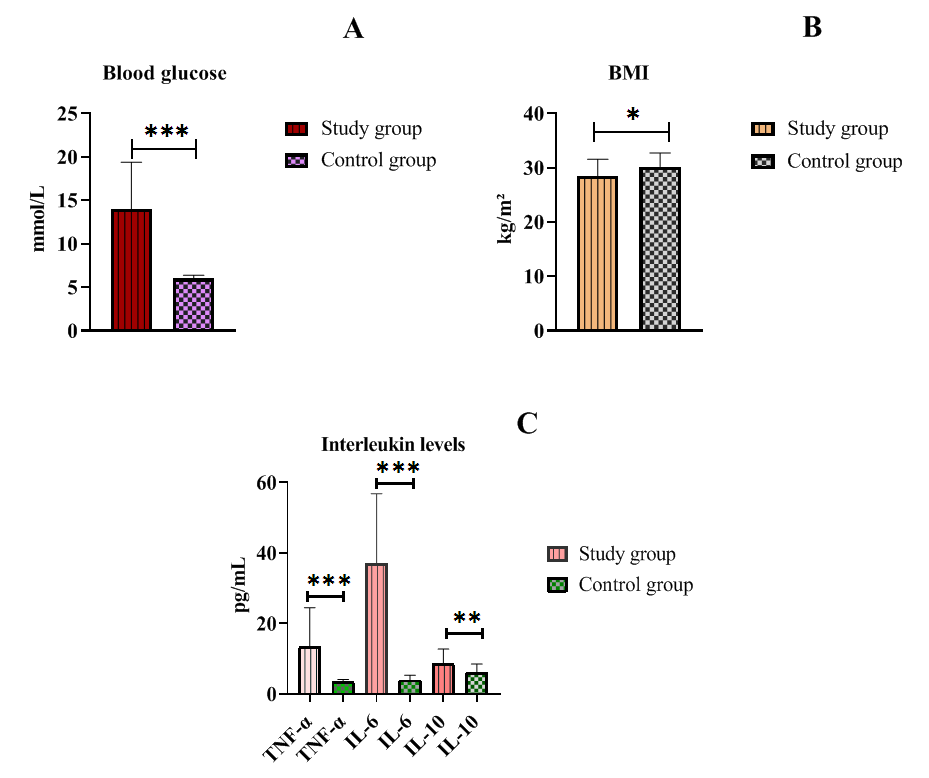 | Figure 1. Glucose, BMI, and Cytokine Levels in Patients with Type 2 Diabetes |
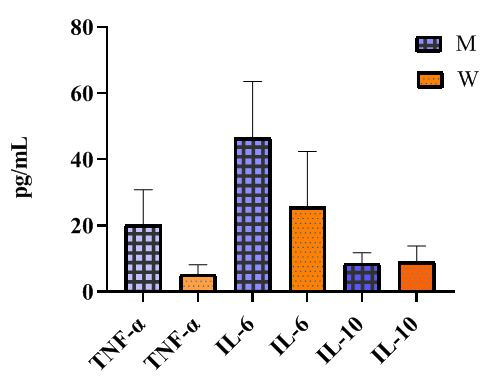 | Figure 2. Gender Differences in Interleukin Levels in Type 2 Diabetes Patients |
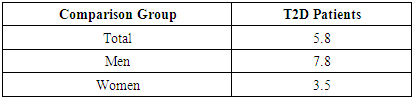
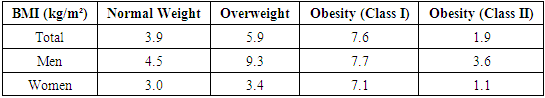
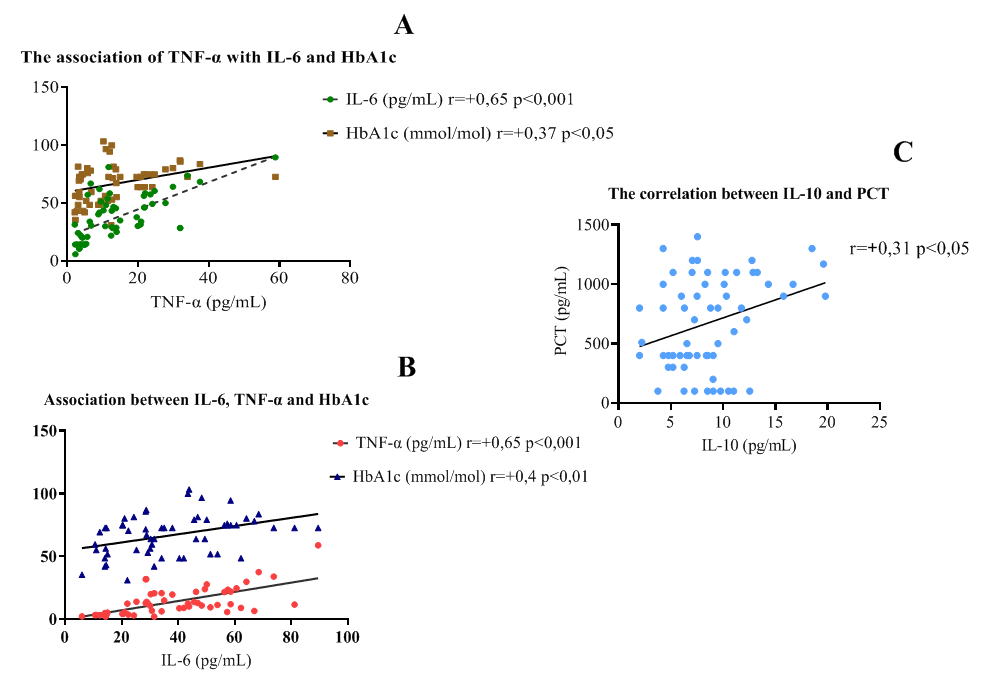
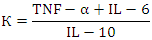 where K represents the sum of pro-inflammatory cytokines TNF-α and IL-6, divided by the anti-inflammatory IL-10 level.This coefficient allows for an integrated assessment of different clinical manifestations of T2D, indicating whether the immune response is skewed toward T-helper (Th1 or Th2) polarization, which is critical for evaluating chronic inflammation severity and the risk of complications in T2D.
where K represents the sum of pro-inflammatory cytokines TNF-α and IL-6, divided by the anti-inflammatory IL-10 level.This coefficient allows for an integrated assessment of different clinical manifestations of T2D, indicating whether the immune response is skewed toward T-helper (Th1 or Th2) polarization, which is critical for evaluating chronic inflammation severity and the risk of complications in T2D.
|
|
|
|
 | Figure 3. Relationship Between Procalcitonin and Other Indicators |
|
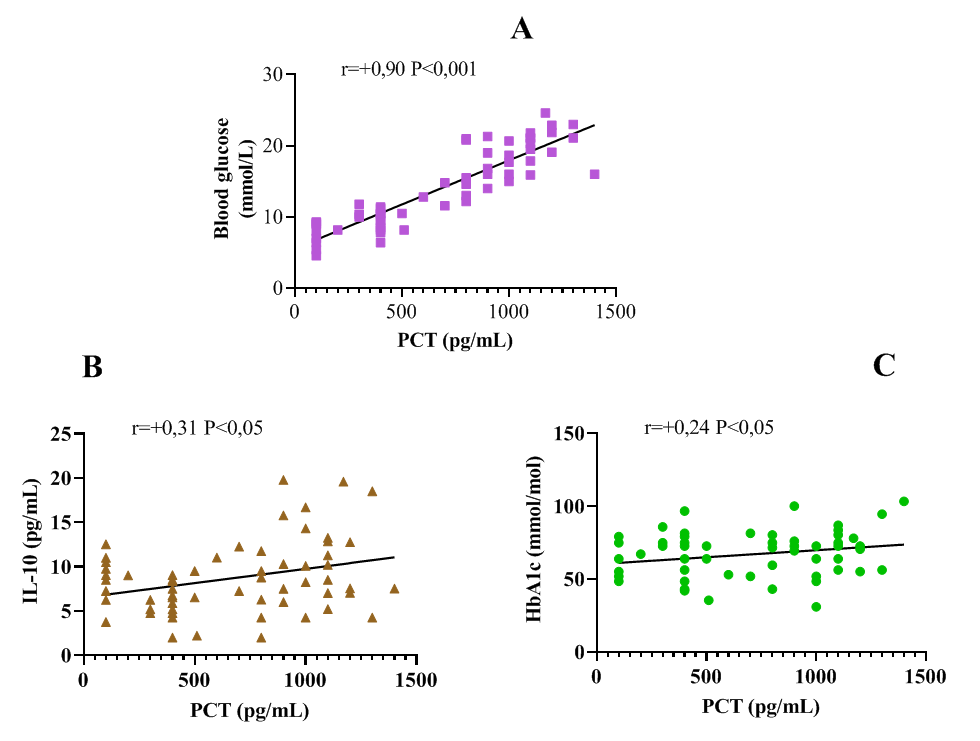
 | Figure 4. Correlation Between Cytokines and Clinical-Laboratory Parameters |
5. Discussion
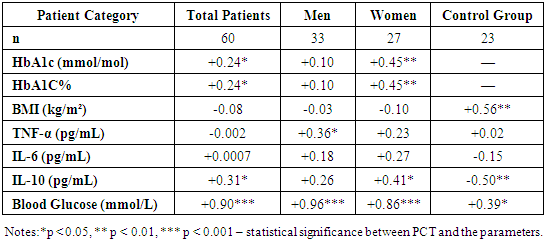
- Despite similar glycated hemoglobin levels, significant differences in TNF-α and IL-6 levels were identified (Table 1). Studies on procalcitonin (PCT) in healthy elderly individuals showed no significant gender-based differences. However, in overweight men, the ratio of pro-inflammatory to anti-inflammatory cytokines was 3.2 times lower than in women. In women, this ratio was elevated even at normal weight, suggesting excessive synthesis of the anti-inflammatory cytokine IL-10. While IL-10 has immunosuppressive properties, in this metabolic syndrome and patient category, it does not fully fulfill this function.An analysis of PCT levels in T2DM patients revealed that women exhibited levels 1.5 times higher than men (P < 0.05). Correlation analysis between the studied parameters showed a strong association between blood glucose levels and PCT (r = +0.96 and r = +0.86 for men and women, respectively). Moderate correlations were identified between TNF-α and PCT (r = +0.36 and r = +0.23 for men and women, respectively). Weak correlations were observed between IL-6 and PCT (r = +0.18 and r = +0.27 for men and women, respectively). Regarding the anti-inflammatory cytokine IL-10, the correlation with PCT was weak in men (r = +0.26) but moderate in women (r = +0.41).These findings reveal gender-specific variations in cytokine interactions. In men, a correlation between TNF-α and PCT was observed, whereas in women, IL-10 was correlated with PCT. No significant correlation was found between PCT and pro-inflammatory cytokines, although a weak correlation was observed between IL-10 and PCT. Literature, data [11-12] on procalcitonin research support the correlation between blood glucose and PCT, consistent with our findings. However, the gender differences in the correlation between PCT and pro- and anti-inflammatory cytokines were identified for the first time.These differences may be explained by calcium metabolism and bone tissue regulation, which are controlled by the parathyroid glands. Their hormone influences calcium mobilization from bone stores, thereby regulating its blood levels. Additionally, calcium levels in bone tissue are affected by calcitonin, secreted by thyroid parafollicular cells, and estrogens, which stimulate calcitonin secretion [13]. In elderly women, metabolic disorders may be linked to calcium loss during post menopause. A major factor contributing to osteoporosis in this group is estrogen and calcitonin deficiency. The relationship between these metabolic changes and immune status indicators highlights profound alterations in the immune system, particularly in cytokine networks.Nutrients and metabolites also play a crucial role in regulating the effector functions of innate immune cells, both in a resting state and during infections. The bidirectional interplay between innate immunity and metabolic pathways forms an immuno-metabolic axis that is highly regulated. Consequently, metabolic disorders such as obesity, T2DM, and non-alcoholic fatty liver disease are characterized by chronic low-grade inflammation, with elevated pro-inflammatory mediators that alter innate immune functions and create a feedback loop leading to excessive inflammation [14].In healthy, lean adipose tissue, M2 macrophages secrete the anti-inflammatory cytokine IL-10 and M2 marker genes, such as arginase-1, fizz1, and Ym1, to maintain adipose tissue homeostasis [15]. In contrast, during obesity, macrophages infiltrate white adipose tissue, promoting insulin resistance by secreting pro-inflammatory cytokines such as TNF-α, IL-6, IL-13, and interferon-gamma (IFN-γ). Obesity also alters macrophage polarization. During obesity, levels of M1 macrophages—known for their pro-inflammatory properties—increase, leading to adipose tissue inflammation and insulin resistance by inhibiting insulin receptor signaling [16-17].Elderly patients with a higher percentage of adipose tissue exhibit significantly greater chronic systemic inflammation than elderly individuals with a normal BMI. This could be due to adipose tissue’s ability to secrete various cytokines, including TNF-α and IL-6, as well as resistin, which can initiate and sustain chronic inflammation and insulin resistance [18-19]. Research on the significance of PCT in T2DM patients with hyperglycemia remains limited. A few experimental studies [8] on animals have also indicated that hyperglycemia and PCT levels are influenced by gender.Thus, our findings suggest significant metabolic disturbances in T2DM that are closely linked to cytokines produced by immunocompetent cells. The cytokine imbalances we identified may serve as early indicators of the risk of T2DM complications, particularly diabetic foot syndrome, allowing for timely preventive measures.
6. Conclusions
- 1. Pro-inflammatory cytokines TNF-α and IL-6 were elevated in elderly T2DM patients compared to control values (P < 0.001). The anti-inflammatory cytokine IL-10 was 1.5 times higher than control levels (P < 0.01). PCT levels were significantly increased in T2DM patients compared to healthy individuals of the same age group.2. TNF-α and IL-6 levels were significantly higher in men than in women, while IL-10 levels showed no significant gender differences.3. A strong correlation was observed between blood glucose levels and PCT in both men and women.4. The findings confirm the role of IL-6 in glucose metabolism disorders and chronic inflammation in T2DM patients. The correlation between IL-6 and HbA1c suggests that IL-6 may influence glycemic control, highlighting its potential as a biomarker for metabolic disorders.5. The correlation between IL-10 and PCT in T2DM patients suggests complex interactions between anti-inflammatory and infection-related inflammatory processes, emphasizing IL-10’s role in immune regulation and systemic inflammation.6. A strong correlation between TNF-α and IL-6 (P < 0.001) indicates a close relationship between these inflammatory mediators, which may play a key role in systemic inflammation and its effects on metabolic processes.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML