-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(6): 1714-1716
doi:10.5923/j.ajmms.20251506.18
Received: May 9, 2025; Accepted: Jun. 2, 2025; Published: Jun. 5, 2025

Effectiveness of Treatment in Patients with Mayer–Rokitansky–Küster–Hauser Syndrome Combined with Ovarian-Origin Hyperandrogenism
Negmadzhanov Bakhodur Boltaevich1, Adilova Madina Niyazovna2, Rabbimova Gulnora Toshtemirovna3
1DSc., Professor, Department of Obstetrics and Gynecology, Samarkand State Medical University, Samarkand, Uzbekistan
2PhD., Associate Professor, Department of Endocrinology, Samarkand State Medical University, Samarkand, Uzbekistan
3PhD., Assistant Professor, Department of Reproductive Medicine, Samarkand State Medical University, Samarkand, Uzbekistan
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Mayer–Rokitansky–Küster–Hauser (MRKH) syndrome is a rare congenital anomaly characterized by uterine and vaginal aplasia in women with a normal 46,XX karyotype. When combined with ovarian-origin hyperandrogenism, it presents additional clinical complexity. This study assessed the clinical and hormonal effectiveness of combined medical and surgical treatments in 66 patients with MRKH syndrome, 30 of whom had signs of hyperandrogenism. The primary treatment included weight reduction, biguanides, and combined oral contraceptives, with some undergoing sigmoid colpopoiesis and ovarian drilling. Results showed significant improvements in clinical symptoms (e.g., acne, hirsutism, unstable blood pressure) and endocrine profiles (e.g., testosterone and prolactin reduction). Ultrasound data revealed decreased ovarian volume and follicle count post-surgery. The Dermatology Life Quality Index (DLQI) improved from 30 to 8 (p<0.05), and overall treatment effectiveness reached 89%. The integration of ovarian drilling with sigmoid colpopoiesis is especially recommended in cases resistant to conservative therapy.
Keywords: MRKH syndrome, Vaginal aplasia, Hyperandrogenism, Ovarian drilling, Sigmoid colpopoiesis, Quality of life
Cite this paper: Negmadzhanov Bakhodur Boltaevich, Adilova Madina Niyazovna, Rabbimova Gulnora Toshtemirovna, Effectiveness of Treatment in Patients with Mayer–Rokitansky–Küster–Hauser Syndrome Combined with Ovarian-Origin Hyperandrogenism, American Journal of Medicine and Medical Sciences, Vol. 15 No. 6, 2025, pp. 1714-1716. doi: 10.5923/j.ajmms.20251506.18.
1. Introduction
- Uterine and vaginal aplasia is a rare congenital anomaly of the reproductive organs characterized by the absence of the uterus and the upper two-thirds of the vagina in women with a normal karyotype (46, XX) [1,4]. This pathology accounts for 5–10% of all genital anomalies [1,4,8]. It is caused by a disruption in the embryonic development of the Müllerian ducts, resulting in the absence of these structures while ovarian function is preserved. The frequency of genitourinary anomalies, including uterine and vaginal aplasia, is increasing and ranks fourth among all congenital malformations, comprising 9.7%, of which 4.0% are related to female reproductive system malformations [1,2].Mayer–Rokitansky–Küster–Hauser syndrome (MRKH) is one of the most well-known forms of uterine and vaginal aplasia. It is characterized by a normal female phenotype, absence of the uterus and vagina (or upper two-thirds of the vagina), and fully functional ovaries [6,7]. The syndrome is named after the scientists who described it: August Mayer, Carl von Rokitansky, Hermann Küster, and Georges Hauser [1,4,8,10].Patients with MRKH experience challenges such as primary amenorrhea, infertility, and significant psychological distress. Although surgical correction of anatomical defects is well-established, data on the combination of MRKH with endocrine and metabolic disorders, particularly ovarian-origin hyperandrogenism, remain limited [2,4,5,6,7,8,11,12].Ovarian-origin hyperandrogenism manifests as excessive androgen production, leading to acne, hirsutism, and menstrual disorders. It is often associated with metabolic abnormalities, including metabolic syndrome, which affects 60–70% of hyperandrogenic patients and significantly worsens their overall health [2,4,5,8-12]. These factors necessitate an individualized approach to diagnosis and treatment.Given the rarity of the combination of uterine and vaginal aplasia with ovarian-origin hyperandrogenism, further research is essential. Optimizing diagnostic and therapeutic strategies for these patients can improve their reproductive and general health, quality of life, and emotional well-being, which is of great clinical importance.
2. Materials and Methods
- A total of 66 patients with vaginal and uterine aplasia were examined and divided into two groups:• Main group (n = 30): vaginal and uterine aplasia combined with signs of ovarian-origin hyperandrogenism.• Comparison group (n = 36): vaginal and uterine aplasia without hyperandrogenism.Patients in the main group received medical therapy (weight loss, biguanides, and combined oral contraceptives). Some patients additionally underwent surgical treatment (sigmoid colpopoiesis + ovarian drilling). The effectiveness of therapy was assessed dynamically based on clinical and laboratory changes.
|
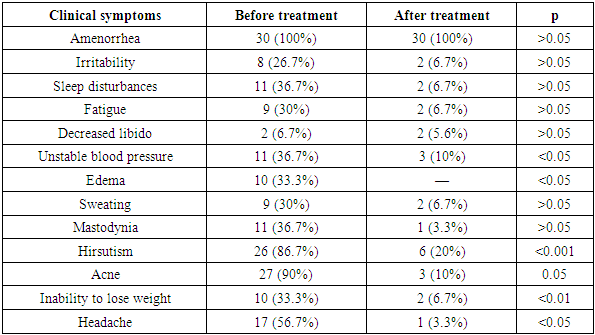
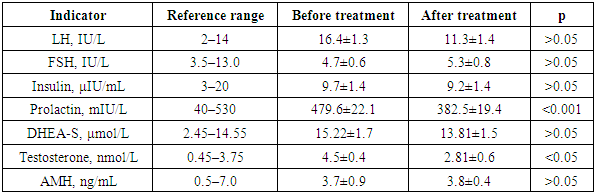
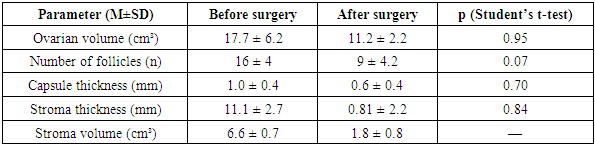
3. Results and Discussion
- Hormonal Profile.
|
|
4. Conclusions
- In patients with vaginal and uterine aplasia combined with ovarian-origin hyperandrogenism, comprehensive therapy (weight reduction, biguanides, COCs, etc.) combined with surgery (sigmoid colpopoiesis and ovarian drilling) demonstrated positive clinical and laboratory outcomes and improved quality of life.Ovarian drilling during sigmoid colpopoiesis is advisable in severe hyperandrogenism resistant to medical therapy and with a high software score (≥12).Drilling promotes ovulation, corrects hyperandrogenism, and may serve as a preparatory stage for assisted reproductive technologies, considering individual characteristics and risks (adhesions, diminished ovarian reserve).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML