-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(6): 1704-1710
doi:10.5923/j.ajmms.20251506.16
Received: May 8, 2025; Accepted: Jun. 1, 2025; Published: Jun. 5, 2025

Prevention, Treatment, and Development of Differential Diagnostic Criteria for Symptomatic Epilepsy in the Early Stages Based on EEG Features and Laboratory Changes in Children with Febrile Seizures
Akhmedova R. Y.1, Sodiqova G. Q.2, Fayzullayev B. R.3
1Assistant of the Department of Neurology, Medical Psychology and Psychotherapy Urgench Branch of the Tashkent Medical Academy, Urgench, Uzbekistan
2Professor, Doctor of Medical Sciences, Department of Neurology, Pediatric Neurology, and Medical Genetics, Tashkent Pediatric Medical Institute, Toshkent, Uzbekistan
3Associate Professor of the Department of Internal Medicine Urgench Branch of the Tashkent Medical Academy, Urgench, Uzbekistan
Correspondence to: Akhmedova R. Y., Assistant of the Department of Neurology, Medical Psychology and Psychotherapy Urgench Branch of the Tashkent Medical Academy, Urgench, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In this article, you can explore the possibility of early prevention and treatment of symptomatic convulsive conditions in children aged 0 to 6 years by summarizing EEG and laboratory changes observed in febrile seizures, as well as by developing differential diagnostic criteria, which may facilitate discussions between pediatricians and neurologists.
Keywords: Febrile seizures, EEG, Hypokalemia, Hypocalcemia, Hypomagnesemia
Cite this paper: Akhmedova R. Y., Sodiqova G. Q., Fayzullayev B. R., Prevention, Treatment, and Development of Differential Diagnostic Criteria for Symptomatic Epilepsy in the Early Stages Based on EEG Features and Laboratory Changes in Children with Febrile Seizures, American Journal of Medicine and Medical Sciences, Vol. 15 No. 6, 2025, pp. 1704-1710. doi: 10.5923/j.ajmms.20251506.16.
1. Introduction
- The aim is to study the peculiarities in the nervous system of children suffering from febrile convulsions, create a differential diagnosis between symptomatic epilepsy and febrile convulsions, and identify and prevent potential risk factors that may occur after febrile convulsions at an early stage.To study the clinical and etiological characteristics of febrile convulsions (FC) in young children and to develop an individual algorithm for diagnosing and conducting dispensary control for this group of patients based on scientific evidence.
2. Materials and Methods
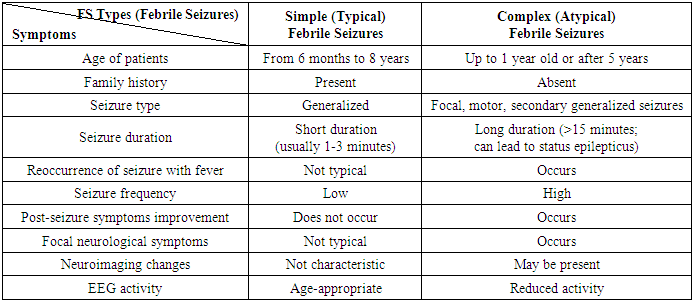
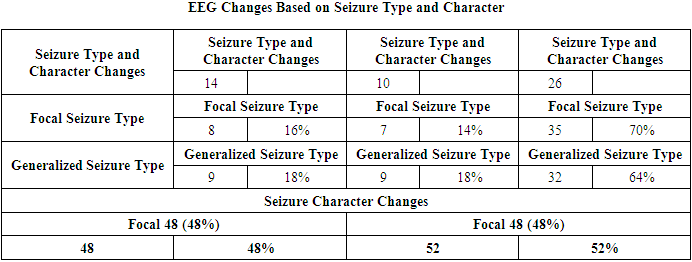
- 100 medical histories of children treated at the Neurology Department of the Multidisciplinary Children’s Medical Center in Khorezm region, Uzbekistan, were analyzed.Scientific significance: According to statistical data, the incidence of febrile convulsions in children has significantly increased. According to data from the United Nations Children's Fund (UNICEF) and the World Health Organization (WHO), the global prevalence of epilepsy is between 5 to 10 per 1,000 people. This rate in Asian and African countries is 3.6 to 4.2 [1]. According to WHO data, in 2010, the number of patients with epilepsy surpassed 50 million. Epilepsy is observed in 0.5-0.75% of the population, while febrile convulsions affect up to 5%. It is reported that 75% of patients do not receive planned treatment. Approximately half of all febrile convulsions occur in children under the age of 15, with the highest frequency observed between the ages of 1 and 9. The condition is more common in males than females. Epilepsy is an age-dependent disease, with epileptic seizures being more common in children and adults over the age of 60. The higher occurrence of convulsive states in children is explained by the unique characteristics of their nervous systems and the numerous causative factors. According to epileptologists, 5% of the global population experiences at least one epileptic seizure in their lifetime. [2,5]Globally, extensive scientific research is being conducted to identify neurological changes in children with epilepsy and febrile convulsions early on and improve the effectiveness of preventive measures. In this regard, studies are being carried out to assess psychoemotional and cognitive disorders in children with febrile convulsions, improve the prevention of neurological complications, optimize treatment, and develop prognostic criteria for the potential development of epilepsy and other diseases after febrile convulsions. Laboratory analyses in children with febrile convulsions, along with the levels of ions such as Ca2+, Mg2+, K+, anemia, or complications in their medical history such as complicated pregnancy or childbirth, play an essential role in predicting the development of neurological complications. Currently, one of the major issues under global attention is whether febrile convulsions are official epilepsy, a transient state, or a syndrome. Febrile seizures are typically paroxysms that occur in tonic or tonic-clonic forms and vary in duration. Febrile convulsions are not classified as official epilepsy, but they attract the attention of pediatricians and neurologists because they can often lead to the development of epilepsy and permanent intellectual and neurological deficits. [3,17]Currently, the issue of febrile convulsions (FC) in children is drawing significant attention from various specialists, including pediatricians, infectious disease specialists, neurologists, and geneticists. In recent years, febrile convulsions have been increasingly referred to as "febrile seizures" rather than "febrile attacks," as the clinical picture of this condition includes not only seizures but also non-convulsive paroxysmal states. According to modern definitions, febrile seizures (FS) are age-related, genetically determined conditions that occur as epileptic seizures in response to high body temperature in the brain. These seizures are observed not only with an increase in body temperature but also during febrile states caused by neuroinfections, and they occur in children between the ages of 3 months and 5 years. The prognosis of the disease is generally positive, but children who have experienced febrile seizures are at higher risk of developing epileptic seizures. [7,8]It is known that for every 1°C increase in body temperature, the intensity of metabolic processes in the brain increases by 7-10%, and therefore the need for oxygen also rises. A gradual increase in body temperature reduces the likelihood of inducing seizures. This is because when body temperature rises slowly, compensatory mechanisms improve cerebral circulation, thereby reducing the risk of hypoxia. Children with perinatal pathology and genetic factors are more prone to febrile seizures. Additionally, disturbances in metabolic processes (such as vitamin D deficiency and hypovolemia), acute infectious processes (such as sepsis, ARVI, pneumonia, and gastrointestinal infections), and endocrine disorders (such as hypoglycemia, hyperglycemia, and diffuse toxic goiter) can also lead to febrile seizures. [4]Febrile seizures are one of the most common convulsions in children between 3 months and 6 years of age. They are considered a convulsive condition that occurs along with a rise in body temperature, in the absence of other causes or diseases (such as central nervous system infections, electrolyte imbalances, or brain injury). [5,9]Currently, 3-5% of the global child population experiences febrile seizures, with rates reaching up to 10% in developed countries. The highest incidence of febrile seizures is observed during the second year of childhood, and 90% of children have their first febrile seizure by the age of 3. Febrile seizures also have a seasonal pattern, similar to some infectious and allergic diseases. Two seasonal peaks are observed: November to January, when upper respiratory viral infections increase, and June to August, when widespread viral gastrointestinal infections occur. In the first case, febrile seizures arise against the backdrop of acute respiratory viral infections (ARVI), and in the second case, functional disturbances of the gastrointestinal system lead to febrile seizures due to the compensatory reaction of the body in response to infection. [10]One of the other problematic aspects of the issue is that the Aral Sea is not only an ecological problem for Central Asia but also for the entire world. This ecological disaster began in the mid-20th century with the disappearance of 90% of the Aral Sea’s water and the formation of desert areas in its place, which marked the onset of a tragic environmental disaster. This disaster is considered an anthropogenic natural ecological catastrophe, and according to many scientists, it is the result of 70% anthropogenic factors. From 1960 to 2009, the area of the Aral Sea shrank from 67,499 km² to 6,700 km². According to data from the World Meteorological Organization, the climate within a 100 km² radius from the sea has changed to a sharply continental climate. This climatic change has led to an increase in the incidence of various diseases among the population living in this area. Specifically, the incidence of respiratory diseases, urinary tract disorders, cancer, as well as anemia and diseases caused by various metabolic disturbances, has been on the rise. Therefore, Khorezm region, being an area affected by this ecological disaster, shows certain unique disease characteristics that differentiate it from other regions of the country. In light of this, we have chosen to analyze and explore the specifics of these diseases and their occurrence in detail.Febrile convulsions (FC) are among the most common convulsions in children aged 3 months to 6 years, observed as a convulsive state associated with an increase in body temperature. [1,5] They occur in the absence of other causes or diseases (such as central nervous system infections, electrolyte imbalances, or brain injuries). Currently, 3-5% of the global child population experiences febrile convulsions, with the incidence reaching up to 10% in developed countries. The highest rate of initial occurrence is observed in the second year of childhood, and 90% of children experience their first febrile seizure by the age of 3. Febrile convulsions also exhibit a seasonal pattern, similar to some infectious and allergic diseases [12,13]. Two seasonal peaks are noted: November to January, when the incidence of upper respiratory viral infections rises, and June to August, when widespread viral gastrointestinal infections occur. In the first case, febrile seizures occur against the backdrop of acute respiratory viral infections (ARVI), while in the second case, functional disturbances of the gastrointestinal system lead to febrile seizures due to the compensatory response of the body to infection [14,17].In the pathogenesis of febrile seizures, the role of metabolic disturbances of certain macro- or microelements may be crucial. It is well-known that the balance of intracellular and extracellular ions in the human body maintains the osmolarity of cells. A change in the levels of calcium, potassium, sodium, and magnesium in the blood can lead to depolarization, which results in conditions like hyponatremia, hypokalemia, and hypomagnesemia, which can further lead to hormonal and even neurophysiological changes [6,12]. These ions are considered the main components of the body's essential hormones (such as TTG, parathyroid hormone, and calcitonin). Research focusing on the neurophysiological functions of sodium, calcium, phosphorus, and magnesium has observed this connection.In addition, any infectious diseases can also trigger febrile seizures (FS) [5]. This is due to the bacteria, viruses, or toxins produced by infectious agents increasing the virulence in the blood, leading to intoxication. This results in the activation of the thermoregulation center in the hypothalamus, which, in response, causes a rise in body temperature. As a result, the bioelectric activity in the brain increases, leading to febrile seizures and the observation of convulsive episodes [6]. Furthermore, febrile seizures are one of the main factors that can occur in infants born to mothers infected with TORCH infections, as well as in those who experienced acute respiratory viral infections during pregnancy [7]. The development mechanism of febrile seizures does not always function the same way, otherwise, every child with a high body temperature would experience a seizure [6]. This suggests that the response of each child’s central nervous system, including the hypothalamus, is influenced by their unique individual physiological structure. Therefore, while some children may not experience a seizure even with a body temperature as high as 40°C, in others with one of the etiological factors mentioned above, febrile seizures can occur even at temperatures of 37.7–38°C. If a child has perinatal brain pathology or genetic factors, hyperthermia generally acts as a trigger for the development of febrile seizures. In each developmental stage, there are specific etiopathogenetic factors that contribute to the development of febrile seizures, particularly febrile convulsions [8].Febrile seizures (FS) can be divided into two groups: [1,2,3]1. Simple FS (SFS) – Short (lasting a few minutes), isolated generalized tonic-clonic seizures, occurring in children aged 3 months to 6 years with febrile temperatures;2. Complex FS (CFS) – Longer lasting (up to 15-20 minutes), recurrent episodes during the day, often with focal components, occurring with subfebrile temperatures, and associated with transient neurological disturbances (post-epileptic).Perinatal factors, especially hypoxia of the brain, developmental anomalies, genetic predisposition, and the anatomical and physiological characteristics of the child's brain play a significant role in the occurrence of this disease in children. These factors enhance brain activity and the susceptibility to seizures, even with minor metabolic changes in the child’s body due to various pathological conditions [9]. There is no specific etiology for the fever that causes febrile seizures, but in 80% of cases, febrile seizures are associated with viral infections, and they are less commonly related to bacterial infections. Roseolovirus is the virus most commonly associated with febrile seizures in the United States and European countries, affecting about one-third of patients under the age of 2 [18,19]. Other studies have shown that febrile seizures associated with Roseolovirus tend to be more complex, often recurring, and may lead to febrile status epilepticus. However, in Asian countries, febrile seizures have been identified as occurring due to influenza A viruses [11,13]. Other viral infections, such as herpes virus (HHV-7), coronavirus (COVID-19), adenovirus, RSV, cytomegalovirus, Shigella, and herpes simplex virus (HSV), have also been proven to cause febrile seizures [16].Vaccines can also increase the risk of febrile seizures, especially a few days after vaccination, where a temporary rise in body temperature may occur. While the risk is low, the likelihood of febrile seizures can be slightly higher if several vaccines are administered simultaneously. In one study, after the combined administration of IPV, PNEUMO, and Penta vaccines, the risk of febrile seizures increased to 30 per 100,000 people, compared to administering them on separate days [15].Febrile seizures can be classified as simple or complex. Simple febrile seizures are generalized tonic or tonic-clonic seizures lasting less than 15 minutes [16,21]. These seizures occur only once within 24 hours in neurologically and developmentally healthy children. If focal characteristics are present, the seizures last longer than 15 minutes [23,24]. If the child has a pre-existing neurological condition, or if the seizures recur (within 24 hours) or occur with a rising temperature, the febrile seizures are classified as complex. The incidence of febrile seizures in Western Europe and the USA ranges from 2% to 5% (Joshi 2005; Waruiru 2004), while in India it ranges from 5% to 10%, in Japan it is 8.8%, and in Guam it is 14% (Waruiru 2004). Data from developing and middle-income countries are insufficient.The role of certain macro and microelements in the metabolic disturbances in the pathogenesis of febrile seizures is considered to be very important [23,24]. Studies on the neurophysiological functions of sodium, calcium, phosphorus, and magnesium show that disturbances in these elements are related to febrile seizures [7]. Furthermore, any infectious disease can also trigger febrile seizures. Genetic factors are also linked to febrile seizures. Anamnesis data show that febrile seizures tend to run in families (familial form of FS) [22,23]. Thus, low levels of calcium, magnesium, and phosphorus in the blood are characteristic of children at risk of recurrent febrile seizures, and this may serve as a criterion for the development of afebrile seizures. Febrile seizures are primary generalized epileptic seizures that manifest as tonic-clonic convulsions. However, they are considered mild epileptic seizures as the prognosis is almost always good. Focal seizures are not typical in febrile seizures. The frequency of febrile seizures decreases as the child gets older. Typically, febrile seizures resolve before the child reaches 6 years of age [5,20,21].To date, EEG (electroencephalography) is the only objective diagnostic method for detecting epilepsy, determining the form of the disease, and distinguishing it from other paroxysmal conditions. EEG data should be considered not only for healthy individuals, especially those working in stressful or harmful production conditions but also when examining patients with various pathological conditions. This is crucial for selecting the most effective treatment methods and monitoring the treatment in the early stages of the disease. EEG allows for tracking the dynamics of the effects of medications and helps assess the speed and completeness of the disappearance of signs of brain dysfunction through re-evaluating EEG. Further development of EEG diagnostic algorithms is important because they assist in applying EEG criteria to monitor treatment effectiveness. Pharmacoelectroencephalographic (pharmaco-EEG) profiles of various drugs are widely used to assess the therapeutic effectiveness of medications and identify new substances. The primary aim of EEG in experimental and clinical studies is to identify specific biomarkers of biochemical changes caused by drug effects. The history of modern clinical pharmaco-EEG begins with the research of Hans Berger, who first recorded changes in human brain bioelectric activity using cocaine, morphine, scopolamine, and chloroform, noting that each chemical caused various changes in the frequency and amplitude of bio-potentials (Berger H., 1929, 1931, 1969). Nowadays, due to the development of new computer technologies, EEG recording and analysis have gained significant time efficiency and spatial resolution, which greatly enhances the capabilities of this method [10].Although EEG changes related to febrile seizures do not have a specific typical pattern, they still show certain individual characteristics. To date, the percentage of epileptiform activity (epileptic activity) associated with febrile seizures is not fully clear. According to data from various authors, this percentage ranges from 2% to 80%, depending on the child’s age and the time after the seizure. It is also known that bioelectric activity slowing (diffuse slowing wave activity, the presence of multiple focal epileptic activity) can persist for up to 7 days after febrile seizures. In complex seizures, EEG has high prognostic value, but there are no clear recommendations regarding the necessity of performing it. Based on our experience, during a seizure, the presence of epileptic activity in the EEG is detected in approximately 52.3% of cases, whereas in the pre-seizure period, this figure decreases to 25.6%. These changes are mostly observed in the temporal (temporal), fronto-temporal (frontal-temporal), and frontal (frontal) regions. Changes in temporal electrodes were noted in 36.6% of cases, in fronto-temporal electrodes in 33.2%, and in frontal electrodes in only 17.75%. In the remaining 13.5% of cases, changes were evenly distributed among other regions, such as central, parietal, and occipital. Pathological activity is mostly asynchronous, observed simultaneously from both the left and right sides. Activity visible from only one side is rare, but secondary bilateral synchronization and secondary synchronization starting from the first temporal region are frequently observed. [4]Thus, based on EEG changes, febrile seizures can be classified into different groups according to the characteristics of convulsions, localization of the epileptic focus, and the EEG frequency. Although EEG changes related to febrile seizures do not have a specific typical pattern, they still show certain individual characteristics. To date, the percentage of epileptiform activity (epileptic activity) associated with febrile seizures is not fully clear [23]. According to data from various authors, this percentage ranges from 2% to 80%, depending on the child’s age and the time after the seizure. It is also known that bioelectric activity slowing (diffuse slowing wave activity, the presence of multiple focal epileptic activity) can persist for up to 7 days after febrile seizures. In complex seizures, EEG has high prognostic value, but there are no clear recommendations regarding the necessity of performing it. Based on our experience, during a seizure, the presence of epileptic activity in the EEG is detected in approximately 52.3% of cases, whereas in the pre-seizure period, this figure decreases to 25.6%. These changes are mostly observed in the temporal (temporal), fronto-temporal (frontal-temporal), and frontal (frontal) regions. Changes in temporal electrodes were noted in 36.6% of cases, in fronto-temporal electrodes in 33.2%, and in frontal electrodes in only 17.75%. In the remaining 13.5% of cases, changes were evenly distributed among other regions, such as central, parietal, and occipital. Pathological activity is mostly asynchronous, observed simultaneously from both the left and right sides. Activity visible from only one side is rare, but secondary bilateral synchronization and secondary synchronization starting from the first temporal region are frequently observed. The incidence of the disease is higher among boys than girls, with a ratio of 2.2:1, indicating that febrile seizures are more common in boys. [20]Based on our observations and the collected data, it is important to note that during the EEG recordings of children with febrile seizures and those with symptomatic epilepsy, no epileptic foci were detected in the brain of children who had increased intracranial pressure and fever, with seizures occurring within the first 24 hours. These children were grouped into the first examination group and designated as the main group, which comprised 76.2% of the cases. The next group was considered the "risk group," which accounted for 24.8% of the cases. This group consisted of children who, based on age and complaints, had not yet been diagnosed with symptomatic epilepsy. However, EEG findings, complaints, and anamnesis indicated that these children required increased monitoring, and preventive measures for fever were recommended. [6]
|
|
|
3. Research Results and Conclusions
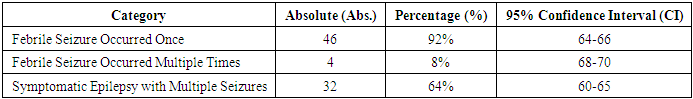
- The observations indicate that 100 children with febrile seizures were classified into five groups based on the etiology. Children under the age of 5 with febrile seizures (FT) during the remission period were included in the study, where febrile seizures gradually decreased by the age of 5-6 years, accompanied by the restoration of normal body function [14].Over several years, children with febrile seizures were observed with constant monitoring, health recovery measures, and ongoing investigations. Specific attention is given to the peculiarities of this region, namely the area surrounding the Aral Sea, which has undergone an ecological disaster. The Aral Sea, located in Central Asia, is one of the world's rapidly shrinking seas. This region experiences higher atmospheric pressure compared to sea level, and the salinity levels are also elevated. Furthermore, the microelement concentrations in the blood of children born in this region are notably different from those of children in other parts of the country, specifically the insufficient levels of essential ions. As a result, the incidence of seizures, particularly febrile seizures, is on the rise year after year.In this regard, children born in this area require increased surveillance and preventative measures to monitor the development of febrile seizures and related neurological issues.Thus, febrile seizures typically present in a generalized tonic-clonic seizure form, which may serve as a criterion for the development of AFS (Acute Febrile Seizures).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML