-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(6): 1675-1678
doi:10.5923/j.ajmms.20251506.09
Received: May 5, 2025; Accepted: May 29, 2025; Published: Jun. 5, 2025

The Role of Citrate Level in Biological Fluids in Early Diagnosis of Congenital Glaucoma
Narzullaeva D. U.1, Bobokha L. Y.2, Buzrukov B. T.3
1PhD, Doctoral Student of the Department of Ophthalmology, Pediatric Ophthalmology, Tashkent Pediatric Medical Institute, Tashkent, Uzbekistan
2Assistant, Ophthalmology, Pediatric Ophthalmology Department, Tashkent Pediatric Medical Institute, Tashkent, Uzbekistan
3Doctor of Medical Sciences, Professor, Head of Department, Ophthalmology, Pediatric Ophthalmology, Tashkent Pediatric Medical Institute, Tashkent, Uzbekistan
Correspondence to: Narzullaeva D. U., PhD, Doctoral Student of the Department of Ophthalmology, Pediatric Ophthalmology, Tashkent Pediatric Medical Institute, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Pediatric glaucoma is one of the most dangerous diseases in pediatric ophthalmologic practice, leading to irreversible loss of vision. Identification of a reliable indicator of glaucoma is in great demand. In recent years, several genetic and non-genetic biomarkers of glaucoma have been described in scientific studies [10,11]. Citrate is one type of organic molecule considered as a biomarker of glaucoma. Objective. To study the role of citrate level in biological fluids as a diagnostic biomarker of congenital glaucoma. Material and methods of the study. The study included 55 children aged from 0 years to 3 years who were under treatment in the eye department of TashPMI clinic. Children were divided into 2 groups: group I (main) included 26 children with confirmed diagnosis of congenital glaucoma; group II (comparison group) included 19 children with cataract diagnosis; group III (control group) included 10 children without ophthalmologic and urogenital diseases. In addition to standard ophthalmologic and instrumental methods of investigation, all children underwent biochemical analysis of blood, urine, and anterior chamber fluid for citrate content (Citric acid). Research results. In patients with glaucoma, the level of citrate in blood averaged 5.0±0.42mmol/l, and in the group of cataract patients was significantly higher 7.2±0.81 (p≤0.05) 1.5 times higher than in the group of glaucoma patients. In the group of glaucoma patients, the urinary citrate level averaged 1.74±0.11 mmol/l, and in the group of cataract patients it was significantly higher 2.18±0.18 (p≤0.05) 1.25 times higher compared to the main group. The level of citrate in intraocular fluid in children with glaucoma was 2.9±0.4, and in the group with cataract it was significantly lower by 1.42 times (2.04±0.25 mmol/l). The presence of a strong inverse reliable relationship between the level of citrate in plasma and LPC indicates that it is sufficient to determine the concentration of citrate only in the blood, as LPC sampling presents certain difficulties. Conclusion. Decrease of citrate level in plasma (0,34±0,07 mmol/l) and urine (0,29±0,06 mmol/l) in children with glaucoma in comparison with the control group (0,69±0,05 and 0,61±0,04 mmol/l, p<0,001) confirms its role as a perspective biomarker for early diagnostics of the disease.
Keywords: Congenital glaucoma, Citric acid, Plasma, Urinary, Aqueous humor
Cite this paper: Narzullaeva D. U., Bobokha L. Y., Buzrukov B. T., The Role of Citrate Level in Biological Fluids in Early Diagnosis of Congenital Glaucoma, American Journal of Medicine and Medical Sciences, Vol. 15 No. 6, 2025, pp. 1675-1678. doi: 10.5923/j.ajmms.20251506.09.
1. Introduction
- Pediatric glaucoma is one of the most dangerous diseases in pediatric ophthalmologic practice, leading to irreversible loss of vision. According to the World Glaucoma Association, pediatric glaucoma is categorized as primary and secondary glaucoma. Congenital and infantile glaucoma together with juvenile open-angle glaucoma constitute primary pediatric glaucoma. Primary congenital glaucoma (PCG) can be further subdivided by age of its onset. PCG with onset from birth to less than 1 month of age is referred to as neonatal/newborn primary glaucoma. PCG with late onset is defined as PCG with onset after 2 years of age. Infantile, if the age of onset is between neonatal/newborn and late glaucoma (i.e., between 1-24 months) [1]. PCG is a rare disease with varying incidence in different countries and ethnic groups. The incidence in Western countries such as Ireland, the UK and the USA is between 1 in 10-20,000 live births [2,3,4,5,6] However, the incidence of PCG is higher in the Middle East, including Saudi Arabia, where close marriages are more common. The estimated incidence of PCG in Saudi Arabia is 1 in 2500 live births [7]. According to the congenital glaucoma registry at King Khaled Eye Specialist Hospital, the Southern region of Saudi Arabia had the highest prevalence of PCG (27.8%), followed by the Western province (23.6%) and the Central region (22.2%). However, the lowest prevalence was recorded in Eastern province (11.1%) and Northern province (9%). A very high incidence was recorded in Slovakia, about 1:1250. According to the American Academy of Ophthalmology, proper glaucoma screening should mainly consist of intraocular pressure (IOP) measurements, visual field testing, evaluation of the optic nerve head (ONH) and retinal nerve fiber layer (RNFL). The reliability and repeatability of visual field testing, IOP measurements, and optic disc scans are important for the diagnosis of glaucoma in children [8,9,10]. However, obtaining reliable and repeatable results of these measurements in children is often a challenge for ophthalmologists [8,9]. Therefore, the identification of a reliable indicator of glaucoma, is in great demand. In recent years, several genetic and non-genetic biomarkers of glaucoma have been described in scientific studies [10,11]. Citrate is one of the types of organic molecules considered as a biomarker for glaucoma. A study in the adult population showed low plasma citrate levels in patients diagnosed with glaucoma, whereas a test in children has not yet been performed. When preparing a test for children, the investigator should consider the idiosyncrasies of certain studies in the pediatric population [12,13,14,15]. Plasma citrate levels as a potential biomarker of glaucoma were first described by Frankl et al. who inadvertently found low citrate levels in patients with glaucoma [12]. The results of this research in a pediatric population are in line with the results obtained by researchers from the University Hospital in Basel. Remer et al, believe that urinary citrate and creatine concentrations at 24-hour urine collection is more appropriate for pediatric population [16]. However, this study obtained similar results in children as those obtained by Frankl et al [12]. Regarding the study group and the control group, no statistical significance was demonstrated between citrate excretion and the ratio between citrate and creatine. In view of the above, it is highly unlikely that renal pathology could affect plasma citrate levels according to Frenkle et al [12]. Thus, citrate can be considered as a universal biomarker of glaucoma.Objective. To study the role of citrate level in biological fluids as a diagnostic biomarker of congenital glaucoma.
2. Material and Methods
- The study included 55 children aged from 0 to 3 years who were treated in the eye department of the TashPMI clinic. The children were divided into 2 groups: group I (main) included 26 children with confirmed diagnosis of congenital glaucoma; group II (comparison group) included 19 children with cataract diagnosis; group III (control group) included 10 children without ophthalmologic and urogenital diseases. All children, in addition to standard ophthalmologic and instrumental methods of investigation, underwent biochemical analysis of blood, urine, and aqueous humor for Citrate content. For quantitative assessment of Citrate level, enzyme-linked immunosorbent assay (ELISA) is used. BIOBASE-Semi Auto Biochemistry Analyzer (SILVER-Plus) was used to determine the level of biological material samples, and Elabscience, USA reagent was used. The inclusion criteria were children diagnosed with primary congenital and infantile glaucoma.Exclusion criteria were as follows: - optic disc disease- active inflammatory eye disease- head trauma - abnormalities in urinalysis - diseases affecting citrate excretion with urine- taking medications that affect renal function.
3. Results of the Research
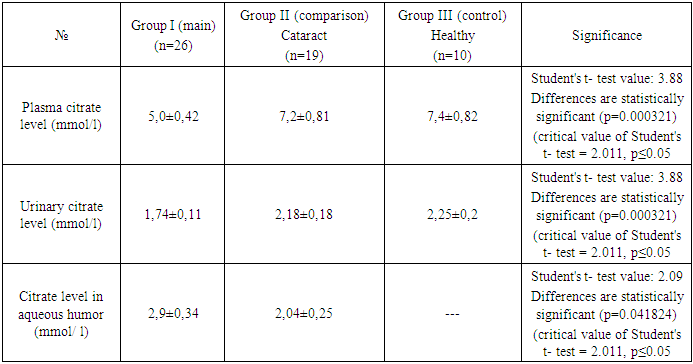
- The blood citrate level in group I patients averaged 5.0±0.42 mmol/l, range varied from 4.2 to 5.6 mmol/l; in group II 7.2±0.81 mmol/l, range varied from 5.4 to 8.0 mmol/l; in III 7.4±058 mmol/l, range varied from 6.5 to 8.0 mmol/l. Urinary citrate levels in patients in groups I II III were distributed as follows: mean values 1.74±0.11; 2.18±0.19 and 2.25±0.15 mmol/l, respectively, ranges ranged from 1.6 to 1.9; 1.9 to 2.5 and 2.0 to 2.4 mmol/l, respectively. The aqueous humor citrate level in group I was 2.9±0.4 mmol/l; the range ranged from 2.2 to 3.4; and in group II, 2.04±0.25 mmol/l; the range ranged from 1.7 to 2.5 mmol/l. The level of aqueous humor citrate in patients III - healthy children was not determined (Table 1).
|
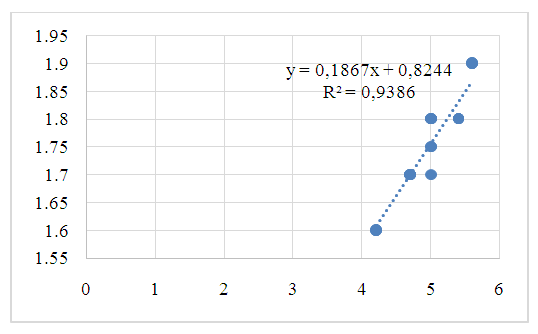 | Figure 1. Pearson correlation of citrate concentration in blood and urine (r - correlation coefficient) in patients of group I |
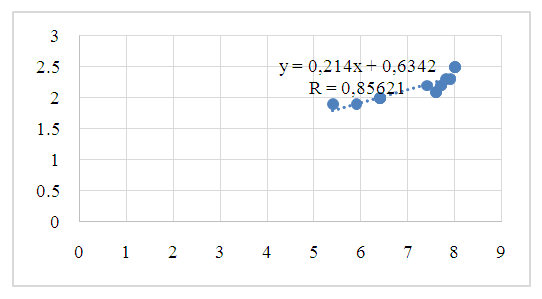 | Figure 2. Pearson correlation of citrate content in blood and urine (r - correlation coefficient) in group II patients |
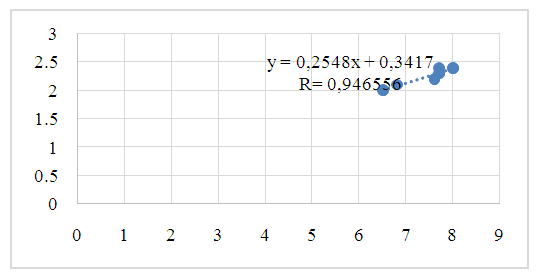 | Figure 3. Pearson correlation of citrate content in blood and urine (r - correlation coefficient) in group III patients |
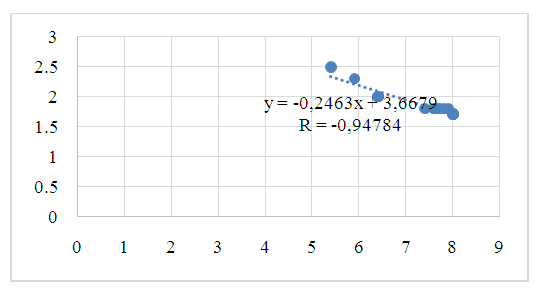 | Figure 4. Pearson correlation of citrate concentration in blood and anterior chamber fluid (r - correlation coefficient) in patients of group I |
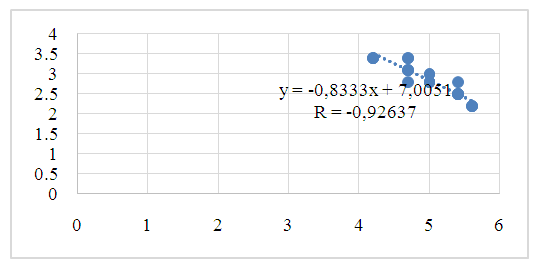 | Figure 5. Pearson correlation of citrate content in blood and aqueous humor (r - correlation coefficient) in patients of group II |
4. Conclusions
- Reduction of citrate level in plasma (0,34±0,07 mmol/l) and urine (0,29±0,06 mmol/l) in children with glaucoma in comparison with the control group (0,69±0,05 and 0,61±0,04 mmol/l, p<0,001) confirms its role as a promising biomarker for early diagnosis of the disease.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML