-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(5): 1575-1584
doi:10.5923/j.ajmms.20251505.59
Received: Apr. 24, 2025; Accepted: May 22, 2025; Published: May 30, 2025

Modern Strategies and International Experience in the Early Diagnosis of Refractive Errors in Pediatric Practice
Iskandarova Shakhnoza Tulkinovna1, Miralimova Malika Mukhammadovna1, Azamatova Fazilat Azamatovna1, Yangieva Nodira Rakhimovna2
1Department of Public Health and Healthcare Management, Tashkent Pediatric Medical institute, Tashkent, Uzbekistan
2Department of Ophthalmology, Tashkent State Dental Institute, Taskent, Uzbekistan
Correspondence to: Iskandarova Shakhnoza Tulkinovna, Department of Public Health and Healthcare Management, Tashkent Pediatric Medical institute, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Refractive errors in children represent a rapidly growing global health challenge, with rising prevalence across all regions and a significant burden on educational and neurodevelopmental outcomes. Early detection is critical for preventing amblyopia, strabismus, and long-term visual impairment. This review provides a comprehensive synthesis of current diagnostic strategies, screening technologies, and international public health initiatives focused on early identification of refractive errors in pediatric populations. Particular emphasis is placed on the integration of instrument-based screening, digital platforms, artificial intelligence, and genomic profiling in early diagnostic frameworks. The article also highlights barriers to equitable implementation, including socioeconomic disparities, technological limitations, and health system fragmentation. By analyzing models from the United States, Europe, and East Asia, as well as global policy recommendations, this review offers evidence-based guidance for optimizing early diagnosis programs in pediatric ophthalmologic care.
Keywords: Pediatric refractive errors, Early diagnosis, Vision screening, Myopia, Public health ophthalmology, Artificial intelligence, School-based eye care
Cite this paper: Iskandarova Shakhnoza Tulkinovna, Miralimova Malika Mukhammadovna, Azamatova Fazilat Azamatovna, Yangieva Nodira Rakhimovna, Modern Strategies and International Experience in the Early Diagnosis of Refractive Errors in Pediatric Practice, American Journal of Medicine and Medical Sciences, Vol. 15 No. 5, 2025, pp. 1575-1584. doi: 10.5923/j.ajmms.20251505.59.
Article Outline
1. Introduction
- Refractive errors in children represent not only a widespread ophthalmological issue but also a critical public health challenge due to their early onset, rapid progression, and long-term impact on visual development, cognitive abilities, and overall quality of life. Myopia, hyperopia, and astigmatism account for the majority of visual impairments in the pediatric population and are often asymptomatic in early stages, leading to delayed diagnosis and irreversible complications such as amblyopia, strabismus, and reduced educational performance if not detected and managed promptly [1], [2], [3].Over the past decade, there has been a dramatic rise in the global prevalence of pediatric refractive errors, with myopia alone approaching epidemic levels in East and Southeast Asia and increasingly affecting children in Europe and North America [4], [5], [6]. The COVID-19 pandemic further exacerbated this trend due to prolonged indoor confinement, increased screen time, and reduced outdoor activity, giving rise to the term "quarantine myopia" [7], [8], [9], [10]. These behavioral and environmental shifts have prompted renewed attention to the urgency of implementing systematic and technologically advanced screening strategies, particularly in early childhood.Timely identification of refractive errors during the critical period of visual system development — typically before the age of seven — is essential for preventing long-term visual disability. Early intervention during this neuroplastic window can reverse amblyogenic factors, enhance binocular vision, and ensure proper visual-cognitive integration [11], [12], [13]. Consequently, numerous international organizations, including the World Health Organization (WHO), International Council of Ophthalmology (ICO), and American Academy of Pediatrics (AAP), emphasize the need for integrating routine vision screening into standard pediatric care [14], [15], [16].Despite the availability of effective diagnostic tools — such as handheld autorefractors, photorefraction, and tele-ophthalmology platforms — global disparities remain substantial. Low- and middle-income countries often face critical barriers such as lack of trained personnel, inadequate infrastructure, and low parental awareness, which hinder the deployment of mass screening programs and follow-up services [17], [18], [19]. These disparities not only limit early detection but also perpetuate inequities in visual health outcomes.Recent advances in artificial intelligence, portable autorefractor devices, smartphone-assisted screening platforms, and cloud-based data systems have significantly expanded the scope and efficiency of early diagnosis. These innovations are now being adopted and evaluated in various international settings, demonstrating promising outcomes in terms of sensitivity, specificity, and cost-effectiveness [20], [21], [22]. Moreover, large-scale multicenter studies and national screening programs, such as the Vision in Preschoolers (VIP) study, EUSCREEN, and campaigns led by the Global Burden of Disease (GBD) initiative, continue to generate high-quality evidence for policy development and clinical practice guidelines [15], [23], [13].This review aims to provide a comprehensive synthesis of current strategies and international experiences in the early diagnosis of pediatric refractive errors. By analyzing diagnostic approaches, screening technologies, epidemiological trends, and implementation models across diverse healthcare systems, this article seeks to offer evidence-based insights and practical recommendations for optimizing pediatric vision care globally.
2. Classification and Etiology of Refractive Errors in Children
- Refractive errors in the pediatric population represent a complex spectrum of ocular developmental disorders, characterized by the eye’s inability to focus light precisely on the retinal plane. The fundamental basis lies in the imbalance between the optical power of the eye and the axial length, resulting in aberrant image formation and compromised visual acuity during critical neurodevelopmental stages [2], [3].
2.1. Classification of Refractive Errors
- Pediatric refractive errors are broadly classified into four main types:• Myopia (nearsightedness) – light focuses in front of the retina due to excessive axial elongation.• Hyperopia (farsightedness) – light focuses behind the retina, typically due to shorter axial length.• Astigmatism – light is focused at multiple points due to irregular corneal or lenticular curvature.• Anisometropia – significant interocular refractive asymmetry, disrupting binocular visual development.Each of these forms has distinct anatomical, functional, and neuro-ophthalmological consequences, particularly during the first decade of life when emmetropization and cortical visual pathways are undergoing refinement [12], [24].
2.2. Myopia: A Developmental and Environmental Phenomenon
- Myopia is currently the most dynamically increasing pediatric refractive disorder globally. In East and Southeast Asia, its prevalence among adolescents has reached pandemic proportions, reflecting a striking shift in the visual ecology of childhood [4], [7]. While axial elongation remains the principal anatomical substrate, recent studies have elucidated multifactorial etiological mechanisms involving:• Genetic susceptibility: over 200 myopia-associated loci identified through GWAS, including variants in PRSS56, ZIC2, and GJD2, which regulate scleral growth and retinal signaling [4].• Environmental influences: prolonged near-work activities, digital screen exposure, urbanized indoor lifestyles, and decreased outdoor time are recognized as key modifiable risk factors [25], [26].• Neurochemical regulation: insufficient exposure to natural light reduces retinal dopamine release, a critical inhibitor of axial growth, thereby accelerating myopization in susceptible children [10], [27].
2.3. Hyperopia: Underrecognized Risk in Early Visual Development
- Hyperopia is physiologically present in infancy but is expected to resolve through emmetropization by the age of 5–6 years. High hyperopia that persists beyond this period is associated with accommodative stress, blurred near vision, and the development of accommodative esotropia and bilateral amblyopia [11], [3]. Unlike myopia, hyperopia is often overlooked due to effective accommodative compensation, underscoring the need for cycloplegic refraction in screening protocols [15].
2.4. Astigmatism: Optical Distortion with Cortical Impact
- Astigmatism results from non-uniform curvature of the cornea or lens and leads to meridional blur, adversely affecting both central and peripheral vision. Its impact is not limited to optics; uncorrected astigmatism in early life interferes with synaptic pruning and orientation-specific neuronal development in the visual cortex, predisposing to meridional amblyopia [6], [24]. The etiology is partially genetic but may also be influenced by eyelid tension and perinatal ocular structure anomalies [28].
2.5. Anisometropia: Amblyogenic Asymmetry
- Anisometropia, often subtle and underdiagnosed, is one of the most potent risk factors for unilateral amblyopia. Even refractive asymmetry as low as 1.00 D can cause persistent suppression of the image from the more defocused eye, particularly in the absence of strabismus [11]. Early detection and prompt refractive correction are paramount for restoring binocular fusion and preventing cortical suppression [3].
2.6. Integrative Etiological Model
- The contemporary etiological model of refractive error formation integrates genetic architecture, structural ocular growth regulation, and sensory-environmental modulation. Genetic predisposition determines baseline ocular dimensions and neurochemical signaling cascades, while environmental exposure modulates gene expression through epigenetic pathways [20], [29].Critically, evidence supports the gene–environment interaction hypothesis, wherein genetic risk alleles amplify susceptibility to adverse environmental exposures. For instance, myopic children with the A allele in GJD2 show significantly greater axial elongation in response to reduced daylight exposure and excessive screen time compared to non-carriers [13].
3. Epidemiology and Global Burden
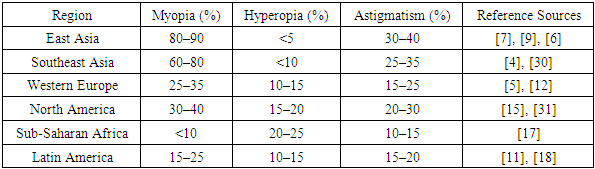
- Refractive errors in children constitute a rapidly growing public health concern, with significant variations in prevalence across different geographic, socioeconomic, and ethnic populations. Epidemiological studies conducted over the past two decades demonstrate a steep rise in the incidence of myopia, in particular, which has reached endemic levels in some urban regions of East and Southeast Asia. According to large-scale population-based cohorts, the prevalence of myopia among adolescents in these areas now exceeds 80–90%, a figure that reflects profound shifts in visual behavior, environmental exposures, and educational demands [4], [7], [9].Moreover, a notable trend is the decreasing age of myopia onset. Whereas myopia previously emerged during adolescence, recent findings indicate its manifestation in early primary school years, which correlates with a longer period of progression and increased risk of high axial elongation and associated complications [30], [26]. In contrast, countries in Western Europe and North America report more moderate prevalence rates, although a clear upward trajectory is evident [5], [12]. Hyperopia and astigmatism remain prevalent globally, especially in preschool-aged children, and frequently go undetected due to compensatory mechanisms or lack of routine screening [15], [3].Socioeconomic disparities play a crucial role in the global burden of refractive errors. In high-income countries, structured vision screening programs, public awareness, and early access to correction significantly mitigate long-term sequelae. In contrast, low- and middle-income regions face persistent challenges related to underdiagnosis, absence of school-based screening, and insufficient ophthalmologic infrastructure, resulting in higher rates of visual disability and academic underachievement [17], [18].The COVID-19 pandemic further accelerated the burden of myopia among children. Periods of school closures, increased screen time, and restricted outdoor activities collectively contributed to a surge in new diagnoses of myopia — a phenomenon now referred to as “quarantine myopia” [10], [9]. Multiple international studies documented statistically significant increases in myopia incidence and axial elongation during the years 2020–2022, especially in children aged 6 to 10 years [7], [20].The table below summarizes the reported prevalence of pediatric refractive errors in various global regions, based on published data from population-based studies.
|
4. Importance of Early Diagnosis
- Early detection of refractive errors in children is a cornerstone of effective ophthalmologic care and a critical determinant of visual, cognitive, and social development in early childhood. Given the heightened neuroplasticity of the visual system during the first decade of life, undiagnosed and uncorrected refractive anomalies—especially in the preschool and early school years—can lead to persistent visual dysfunction, amblyopia, strabismus, and deficits in binocular integration [3], [28], [24]. These complications, while preventable, are often irreversible if refractive errors are not identified and corrected within sensitive periods of cortical visual maturation.Numerous neurodevelopmental studies confirm that visual input during infancy and early childhood plays a pivotal role in shaping the architecture of the visual cortex. In cases of uncorrected anisometropia or significant astigmatism, abnormal or asymmetric stimulation of the retina leads to cortical suppression of the blurred image, with consequent failure to develop normal synaptic connections in the striate cortex [6], [12], [2]. This suppression can persist even after optical correction if initiated too late, emphasizing the need for vision screening during the emmetropization phase, ideally before age 5 [15], [5].Beyond the structural and sensory consequences, unrecognized refractive errors are increasingly recognized as a significant contributor to poor academic performance, reduced attention span, and social disengagement. Children with hyperopia or mixed astigmatism frequently experience difficulty with sustained near work, reading, and visual tracking, leading to behavioral manifestations that may be misinterpreted as attention-deficit hyperactivity disorder or learning disabilities [11], [13]. Corrective intervention, in many such cases, results in marked improvements in educational functioning and psycho-emotional stability, without the need for pharmacological or psychiatric measures [15], [3].Of equal concern is the rapid progression of myopia in cases where diagnosis is delayed. Uncorrected myopia is not a benign condition; rather, it is associated with progressive axial elongation, increased risk of retinal detachment, macular degeneration, choroidal neovascularization, and irreversible vision loss in adulthood [8], [27]. Emerging evidence confirms that early detection and optical intervention—including spectacle correction, low-dose atropine, and orthokeratology—can significantly reduce axial growth and limit the long-term morbidity of high myopia [20], [14], [29].From a systems perspective, the failure to diagnose refractive errors at an early stage places a substantial burden on healthcare and educational infrastructure. In many countries, including low- and middle-income settings, the cost of vision-related educational delays, special-needs resources, and rehabilitation exceeds the modest investment required for early screening and correction [17], [18]. Vision screening at age-appropriate intervals is now recognized not only as a clinical necessity but as a public health imperative with broad implications for national productivity and social equity [4], [16].Technological advancements have enabled the implementation of vision screening even in resource-limited environments. Portable autorefractors, wavefront analyzers, and smartphone-assisted screening platforms offer new avenues for early identification of at-risk children, especially when deployed through school health programs or community-based outreach [21], [20], [22]. Moreover, international models such as the VIP and EUSCREEN studies have demonstrated the feasibility and cost-effectiveness of large-scale screening initiatives in diverse educational and cultural contexts [15], [23].Finally, early diagnosis serves as the gateway to modern individualized care. Genetic predisposition to refractive error—particularly myopia—can now be incorporated into screening algorithms, facilitating risk stratification and early preventive counseling [4], [32], [10]. In this context, the integration of ophthalmology with pediatrics, genetics, and education constitutes a new paradigm in child health, one that acknowledges vision not only as a sensory function but as a determinant of developmental potential and lifelong well-being.
5. Current Strategies for Screening and Diagnosis
- The successful implementation of early detection strategies for pediatric refractive errors requires not only diagnostic accuracy but also scalability, cost-efficiency, and adaptability to varying healthcare environments. Over recent decades, substantial progress has been made in screening methodology — shifting from reliance on purely clinical, subjective tests toward portable, automated, and technology-integrated systems designed for mass application, particularly in early childhood [15], [6], [20].
5.1. Traditional Methods: Clinical Simplicity with Limitations
- Basic visual acuity testing, using age-adjusted optotypes such as LEA symbols or HOTV charts, remains the most widely used method in primary care and school-based screening programs. While these tests are low-cost and easy to administer, especially for children aged 4 years and older, their diagnostic utility is limited by factors such as poor cooperation, inability to detect latent hyperopia or astigmatism, and examiner dependency [11], [3].Cycloplegic autorefractometry, often considered the gold standard in pediatric refraction, provides highly accurate and reproducible data. However, its use in mass screening is restricted by the need for pharmacologic cycloplegia, longer examination time, and medical personnel trained in pediatric eye care [12], [13]. Thus, while valuable in confirmatory diagnosis, its role in population-wide screening remains limited to selected settings.
5.2. Instrument-Based Screening: A Paradigm Shift
- In response to the shortcomings of traditional approaches, the past decade has witnessed a rapid proliferation of instrument-based screening technologies, particularly those using photorefraction and wavefront analysis. Devices such as Plusoptix, Spot Vision Screener, and Welch Allyn SureSight enable non-invasive, rapid, and child-friendly screening, even in preverbal or non-cooperative patients [6], [31].These tools are especially effective in detecting amblyogenic risk factors — including anisometropia, high hyperopia, and strabismus — and have shown consistently high sensitivity and specificity in large-scale validation studies. Their minimal dependence on subjective responses and rapid acquisition time makes them ideal for integration into preschool and community health programs [15], [20].Handheld autorefractors have further improved accessibility, enabling outreach services to reach remote or under-resourced regions. Although cost and calibration requirements remain challenges, they offer a reliable alternative to conventional tabletop refractors in mobile screening initiatives [22], [18].
5.3. Digital and Mobile Platforms: Expanding the Frontier
- Technological innovation has introduced smartphone-based screening solutions, such as GoCheckKids and Peek Acuity, which leverage mobile cameras, integrated applications, and cloud-based data analysis to screen and triage pediatric visual disorders. These platforms facilitate teleophthalmology workflows, data storage, and instant referrals — features particularly valuable in rural, underserved, or post-pandemic settings where traditional infrastructure is limited [21], [22].Although still in the validation phase in many regions, preliminary studies report promising levels of sensitivity (70–90%) and high user acceptability. These tools represent a paradigm shift toward democratization of eye care and integration into primary pediatric practice [20], [16].
5.4. Comparative Summary of Methods
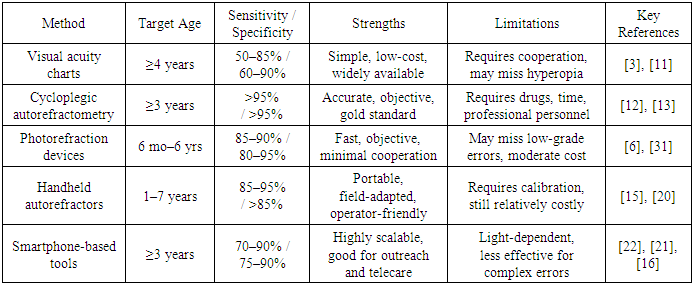
- To highlight the strengths and limitations of the most common diagnostic approaches, the table below presents a structured comparison of key pediatric screening methods currently in use worldwide.
|
5.5. Integrating Technology into Public Health Strategies
- The incorporation of these methods into tiered screening models — where initial visual acuity tests are followed by automated objective methods for high-risk or borderline cases — has been endorsed by multiple international bodies, including the WHO and ICO [15], [27], [33]. This layered approach ensures maximal coverage while optimizing resource allocation and reducing over-referral or underdiagnosis.Countries with successful implementation models often rely on public-private partnerships, community health workers, and digital data tracking to improve follow-up, reduce dropout, and integrate screening with educational and developmental services [17], [18].In conclusion, the evolution of pediatric vision screening is defined by technological innovation, cross-sector collaboration, and an increasing emphasis on universal accessibility. While no single method offers a universal solution, strategic combination and context-specific adaptation of available tools enable effective, scalable, and sustainable early diagnosis programs for refractive errors in children.
6. Importance of Early Diagnosis
- The growing prevalence of refractive errors among children has led to the development of diverse vision screening programs across countries, each adapted to their specific demographic, economic, and healthcare systems. Despite regional differences, the underlying goal remains the same: early identification and timely intervention to prevent long-term visual disability. This section presents key international models and consensus guidelines that form the basis of global pediatric refractive error control strategies.
6.1. United States: Vision in Preschoolers (VIP) and National Guidelines
- In the United States, the Vision in Preschoolers (VIP) Study has played a foundational role in shaping pediatric vision screening protocols. Sponsored by the National Eye Institute, this multicenter project evaluated the diagnostic effectiveness of various screening tools for detecting amblyopia risk factors in 3- to 5-year-old children [11], [15]. The study confirmed that non-specialist-administered, instrument-based methods (e.g., handheld autorefractors and photorefraction devices) provided excellent sensitivity and specificity, establishing them as viable tools for community-level implementation.The American Academy of Pediatrics (AAP), in collaboration with the American Academy of Ophthalmology (AAO) and the American Association for Pediatric Ophthalmology and Strabismus (AAPOS), recommends vision screening at well-child visits starting at age 3, using instrument-based screening when available [3], [14].
6.2. Europe: EUSCREEN and National School-Based Programs
- European countries have adopted varying models of school-based screening, with a strong emphasis on integration into national education and health systems. The EUSCREEN project, supported by the EU Horizon 2020 initiative, has developed comparative models for vision and hearing screening across 41 countries [23]. Its findings underscore the heterogeneity of implementation in terms of age at screening, responsible personnel (nurses, pediatricians, orthoptists), and diagnostic criteria.For example, the Netherlands implements universal vision screening at 6 months, 3 years, and again at school entry, coordinated by youth health services. Germany and the UK focus on school entry vision assessments, with high coverage and referral systems in place [15], [5].The European approach emphasizes early detection, low-cost standardized tools, and strong referral feedback loops, ensuring that children diagnosed with visual issues receive timely corrective treatment.
6.3. East and Southeast Asia: Proactive National Campaigns
- Countries such as China, Singapore, Japan, and South Korea have responded to the myopia epidemic with nationwide screening and prevention campaigns. In China, large-scale vision screening is conducted in schools biannually, with results integrated into national health databases [7], [9]. Myopia is formally classified as a public health priority, and policies actively limit near-work hours and encourage outdoor time in schools [8], [20].Singapore implements a comprehensive program under its School Health Services, combining annual vision screening with optometric referral and myopia education campaigns. The success of these models is attributed to governmental commitment, high parent engagement, and continuous policy revision based on epidemiological data [4], [10].
6.4. Global Guidelines: WHO, ICO, and Expert Consensus
- The World Health Organization (WHO) and the International Council of Ophthalmology (ICO) have issued global action plans emphasizing the early identification of visual disorders in children as part of universal eye health initiatives. Their recommendations call for integration of vision screening into primary healthcare, school entry examinations, and routine pediatric evaluations, with flexibility to adapt tools to local needs and resource availability [16], [27], [34].The 2019 WHO report on "World Vision Health" identifies school-aged vision screening as one of the most cost-effective interventions in global child health, especially in low- and middle-income countries [18], [17], [35].Furthermore, recent expert consensus highlights the value of tiered screening approaches, combining subjective and objective methods, as well as the need for digital recordkeeping, parental education, and cross-sector collaboration to improve follow-up and treatment adherence [14], [29], [36].
7. Challenges and Limitations
- While the global discourse around pediatric refractive error screening has evolved toward evidence-based, technologically supported, and policy-integrated models, the translation of these principles into practice remains fraught with multifactorial limitations. These constraints affect both high-income and resource-limited settings, albeit in different forms and magnitudes. A realistic assessment of these challenges is essential for the design of effective, sustainable, and equitable screening systems.
7.1. Structural and Infrastructure-Related Barriers
- One of the most persistent challenges in implementing vision screening programs lies in the lack of necessary infrastructure. In many low- and middle-income countries, pediatric eye health is not integrated into primary care services. Clinics may lack autorefractors, cycloplegic agents, calibrated acuity charts, and reliable electricity or internet for cloud-connected diagnostic platforms [17], [20], [37].Moreover, even in well-equipped systems, logistical difficulties such as irregular calibration, equipment malfunction, and lack of technical support compromise the continuity and quality of mass screening efforts. These limitations are particularly problematic in rural or nomadic populations, where consistent programmatic presence is often absent [22], [18], [38].
7.2. Workforce Deficiencies and Uneven Specialist Distribution
- The shortage of trained professionals in pediatric vision screening — including ophthalmologists, optometrists, orthoptists, and trained nurses — poses a critical barrier. Many primary healthcare workers are not familiar with the subtleties of pediatric refractive error detection and management, leading to under-referral or misclassification [11], [12].Moreover, even in countries with advanced eye care systems, there is often a geographical imbalance in specialist distribution, with urban areas being overserved and rural regions left without access to basic diagnostic services [23], [5], [39].
7.3. Policy Fragmentation and Lack of Standardization
- An often-overlooked limitation is the fragmentation of vision screening policies. In the absence of unified national frameworks, different regions or institutions may adopt heterogeneous protocols, age thresholds, or referral criteria. This lack of standardization leads to inconsistencies in diagnostic accuracy, follow-up adherence, and treatment coverage [15], [27], [40].Furthermore, integration between health and education sectors remains weak in many countries. School-based screenings are not always linked to clinical services, and there is little longitudinal data tracking of children who fail screenings but do not receive care — creating a "diagnostic drop-out" phenomenon [13], [3], [41].
7.4. Sociocultural and Economic Barriers at the Family Level
- Parental factors — including health literacy, socioeconomic status, cultural attitudes, and psychological readiness — significantly influence whether a child receives timely and appropriate vision care. In some cultures, spectacle wear is stigmatized; in others, parents may mistakenly believe that children “will outgrow” vision problems [9], [31], [42].Cost is another critical issue. Even when screening is free, the subsequent corrective measures — including eyeglasses, pharmacological therapy, or clinical referral — may not be covered or may be unaffordable for families, especially in uninsured or informal labor populations [29], [15], [43].Limited awareness of the long-term developmental consequences of uncorrected refractive errors further exacerbates non-adherence to follow-up. Without targeted parental education, even technically successful screening campaigns may have suboptimal impact [13], [18], [44].
7.5. Diagnostic and Technological Limitations
- Although many modern screening tools demonstrate high sensitivity and specificity, none are without limitations. Visual acuity charts are poorly predictive of hyperopia and often miss astigmatism. Photorefraction devices may underperform in high ambient light or with non-central fixation. Smartphone-based applications are highly operator-dependent and lack validation in children under 3 years of age [6], [21], [31].Additionally, newer technologies are often not interoperable with national health information systems, limiting data aggregation, audit, and longitudinal follow-up — all of which are essential for policy feedback and program refinement [20], [22], [45].
8. Future Perspectives and Innovations
- The paradigm of pediatric refractive error screening is undergoing a profound transformation, driven by the convergence of technological innovation, evidence-based public health policy, and a growing recognition of the long-term cognitive, social, and economic consequences of uncorrected visual impairment in childhood. Future directions in this field are no longer limited to improving diagnostic accuracy alone but increasingly involve integrative, predictive, and equity-oriented strategies aimed at transforming early diagnosis into a sustainable pillar of pediatric care [5], [14], [13].
8.1. Artificial Intelligence and Predictive Analytics
- The incorporation of artificial intelligence (AI) and machine learning algorithms into pediatric ophthalmology has already demonstrated significant potential to revolutionize early screening. Deep-learning-based systems trained on thousands of labeled retinal images can now accurately detect amblyogenic risk factors, refractive asymmetries, and axial elongation patterns with diagnostic accuracy rivalling that of human experts [20], [22], [21].AI-based models are also being developed to incorporate behavioral and demographic predictors — such as screen time, near work, family history, and urban living — to generate real-time individual risk profiles for myopia progression and early-onset astigmatism [8], [4], [32]. These tools, once integrated into mobile platforms, will allow for personalized visual surveillance and timely intervention.
8.2. Teleophthalmology and Remote Population Coverage
- The emergence of telemedicine, particularly during and after the COVID-19 pandemic, has proven indispensable in expanding access to early vision screening in underserved regions. Smartphone-based autorefractors, photorefraction tools, and cloud-integrated diagnostic apps (e.g., Peek Vision, GoCheckKids) have shown high feasibility for large-scale deployment in schools and rural areas, offering reliable preliminary assessment with minimal professional input [7], [6], [17].These platforms enable real-time data transmission, cloud-based archiving, and automated referral generation — significantly reducing the diagnostic gap between urban and rural populations and ensuring timely follow-up for children at risk [15], [22], [46].
8.3. Genomic Insights and Personalized Risk Stratification
- Advances in ocular genomics are opening a new chapter in pediatric vision science. Genome-wide association studies have identified over 200 loci linked to refractive development, including PRSS56, GJD2, and ZIC2, which influence scleral remodeling and retinal signal processing [4], [10], [32].The future integration of genetic risk profiling into pediatric screening protocols holds promise for early identification of children predisposed to progressive myopia or congenital refractive anomalies. When combined with AI and behavioral data, these models will enable precision screening, where high-risk individuals receive tailored monitoring, lifestyle modification counseling, and early therapeutic interventions [26], [13], [47].
8.4. Digital Public Health Ecosystems
- For early diagnosis strategies to achieve long-term impact, they must be embedded within robust national digital health infrastructures. Countries leading in pediatric vision care — including Singapore, the Netherlands, and China — have developed interoperable electronic medical records that integrate vision screening data with school health systems and pediatric primary care [5], [16], [48].Such infrastructures facilitate longitudinal tracking, reduce loss to follow-up, and support data-driven health policy, allowing real-time adjustments to screening protocols based on population-level epidemiological trends [27], [20], [49]. Moreover, linking screening outcomes to electronic alerts, parental education portals, and teleconsultation services greatly enhances program adherence and efficiency.
8.5. School-Centered and Multisectoral Integration
- Although Moving forward, early diagnosis of refractive errors must be reframed as an educational and developmental imperative, not merely a clinical task. Vision screening should be institutionalized within national education policies, with mandatory inclusion at school entry and periodic reassessment throughout the primary cycle [3], [50], [51].This will require coordinated policy frameworks involving ministries of health, education, and finance, along with strategic partnerships with non-governmental organizations and local health workers. Cross-sector collaboration will be key to overcoming the barriers outlined previously — including low parental awareness, diagnostic dropout, and inequality of access [18], [29], [52].In parallel, teachers and school administrators should be empowered to identify early signs of visual difficulty and facilitate communication with parents and health authorities. Integrating screening with broader child development assessments also allows early identification of neurodevelopmental or learning-related comorbidities associated with uncorrected vision problems [12], [15].
9. Conclusions
- Early detection of refractive errors in children is no longer a matter of isolated clinical decision-making but a global public health priority with profound implications for education, neurodevelopment, and health equity. The last two decades have witnessed a substantial shift toward the development of structured, evidence-based, and technologically enhanced screening programs, many of which have demonstrated high diagnostic accuracy, feasibility, and cost-effectiveness in diverse populations.International experience clearly shows that timely identification of visual disorders is achievable through a combination of clinical standardization, innovative digital tools, and multisectoral coordination. However, challenges remain — particularly in ensuring equitable access, consistent follow-up, and integration into national health and education systems.Future strategies must prioritize scalable, data-driven, and personalized approaches — including artificial intelligence, genomic risk profiling, and teleophthalmology — while reinforcing the critical role of schools, parents, and primary healthcare providers in early intervention.Ultimately, optimizing the early diagnosis of refractive errors is not only a scientific or technical task; it is a societal responsibility that directly influences the cognitive potential, academic achievement, and lifelong opportunities of millions of children worldwide.
ACKNOWLEDGEMENTS
- The authors express their gratitude to the Department of Public Health and Healthcare Management of Tashkent Pediatric Medical Institute and the Department of Ophthalmology of Tashkent State Dental Institute for their academic support during the preparation of this manuscript.
DISCLOSURE
- The authors declare that there is no conflict of interest regarding the publication of this article. No external funding or expert review disclosures were involved in the preparation of this manuscript.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML