-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(5): 1570-1574
doi:10.5923/j.ajmms.20251505.58
Received: Apr. 21, 2025; Accepted: May 16, 2025; Published: May 30, 2025

Systemic Effects of Acute Renal Ischemia Alterations in Blood Proteins and Hormonal Regulation
Arsenova M. A.1, Iriskulov B. U.2, Akhmedova D. B.2
1Tashkent International Chemical University, Uzbekistan
2Tashkent Medical Academy, Uzbekistan
Correspondence to: Arsenova M. A., Tashkent International Chemical University, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: Acute renal ischemia triggers a complex systemic response involving metabolic, inflammatory, and neuroendocrine mechanisms. These responses significantly impact blood protein composition and hormonal balance, contributing to the progression of kidney injury and systemic dysfunction. Objective: This study aimed to investigate changes in blood protein fractions and stress hormone levels in a rat model of acute renal ischemia. Methods: The experiment was conducted on 24 male Wistar rats divided into control and experimental groups (6 animals per group). Acute renal ischemia was induced by immobilization of the right renal artery and daily subcutaneous administration of adrenaline. Blood samples were collected on days 1, 3, 7, 10, and 14. Total protein and protein fractions were analyzed using an automatic biochemical analyzer. Serum levels of cortisol and adrenaline were measured by ELISA. Results: A significant decrease in total protein (up to 31%) and albumin (up to 30%) was observed, alongside an increase in α1- and β-globulins, indicating an acute-phase inflammatory response and a shift in liver function. A marked rise in cortisol and adrenaline levels (up to 12- and 7.9-fold, respectively) reflected strong and prolonged activation of the HPA axis and the sympathetic-adrenal system. Conclusion: Acute renal ischemia induces pronounced metabolic and hormonal changes, characterized by hypoproteinemia, activation of acute-phase proteins, and elevated stress hormone levels. These findings underline the importance of protein and hormonal markers in understanding the systemic response to renal ischemia and may contribute to the development of diagnostic and therapeutic strategies.
Keywords: Acute renal ischemia, Protein fractions, Cortisol, Adrenaline, Systemic inflammation, HPA axis, Acute-phase response, Rats
Cite this paper: Arsenova M. A., Iriskulov B. U., Akhmedova D. B., Systemic Effects of Acute Renal Ischemia Alterations in Blood Proteins and Hormonal Regulation, American Journal of Medicine and Medical Sciences, Vol. 15 No. 5, 2025, pp. 1570-1574. doi: 10.5923/j.ajmms.20251505.58.
Article Outline
1. Introduction
- Acute renal ischemia triggers a complex systemic response involving metabolic, inflammatory, and endocrine pathways. The kidneys play a central role in maintaining homeostasis, and their dysfunction rapidly affects multiple physiological systems [1]. One of the earliest manifestations of ischemic injury is a disruption in protein metabolism, reflected in significant changes in blood protein composition. Hypoproteinemia, decreased albumin levels, and alterations in globulin fractions are common during acute ischemia, indicating a shift in liver function toward the synthesis of acute-phase proteins and a redistribution of plasma proteins due to increased vascular permeability [2].Simultaneously, activation of the sympathetic-adrenal system and the hypothalamic-pituitary-adrenal (HPA) axis leads to a marked rise in stress hormones such as cortisol and adrenaline. These hormones play a key role in mobilizing energy resources, modulating immune responses, and regulating protein catabolism. However, prolonged hormonal activation may contribute to tissue damage, immunosuppression, and impaired recovery [3-5].Understanding the systemic response to acute renal ischemia is essential for identifying early biomarkers of disease progression, potential therapeutic targets, and strategies to mitigate the impact of systemic inflammation and metabolic imbalance. Despite its importance, the integrated dynamics of serum protein changes and neuroendocrine activation in the context of renal ischemia remain insufficiently studied [6]. This research aims to fill this gap by analyzing the temporal patterns of blood protein fractions and stress hormone levels, providing insights into the adaptive and pathological mechanisms triggered by acute renal ischemia.Acute renal ischemia represents a complex physiological response to various insults such as ischemia, toxins, or trauma, which can lead to significant alterations in metabolic, inflammatory, and hormonal pathways. The kidneys play a critical role in maintaining homeostasis by regulating fluid, electrolyte balance, and protein metabolism [7,8]. When subjected to ischemia, renal function is compromised, leading to a cascade of systemic effects that include changes in blood protein composition and the activation of stress hormones.Proteins in the blood [9], particularly albumin and globulins [10], are essential for maintaining osmotic pressure, immune responses, and transport functions [11]. Acute renal ischemia often results in a reduction of total protein and albumin levels, a phenomenon associated with increased vascular permeability, protein leakage, and decreased liver synthesis of key plasma proteins. In contrast, certain globulin fractions, such as α1-globulins, can increase as part of the acute-phase response, reflecting the systemic activation of inflammation [12].The neuroendocrine response to renal ischemia involves the activation of the sympathetic-adrenal system and the hypothalamic-pituitary-adrenal (HPA) axis. Adrenaline and cortisol, the primary hormones involved in the stress response, are essential for mobilizing energy reserves and modulating immune responses [13]. However, prolonged or excessive activation of these pathways can contribute to tissue damage, immunosuppression, and metabolic imbalances.Understanding the systemic response to acute renal ischemia, including alterations in protein profiles and hormonal regulation, is crucial for developing effective biomarkers for early diagnosis and potential therapeutic targets. While much is known about isolated aspects of the stress response, the integrated dynamics of protein and hormonal changes during acute kidney injury remain poorly understood [14].
2. Purpose of the Research
- This study aims to investigate the systemic response to acute renal ischemia by analyzing changes in blood protein composition and hormonal profile. Specifically, it seeks to assess alterations in total protein and globulin fractions, examine the dynamics of stress hormones (cortisol and adrenaline), and explore the role of acute-phase proteins and inflammatory mediators. The study aims to enhance understanding of the metabolic and neuroendocrine mechanisms involved in acute kidney injury and their potential implications for diagnosis and treatment.
3. Materials and Methods
- Animals and Experimental Design.The study was conducted on 24 male non-inbred rats, with 6 rats per group. The animals were randomly divided into two groups: the experimental group and the control group (intact). All rats were housed under standard conditions (temperature 22-24°C, 12-hour light/dark cycle) with free access to food and water. The study followed ethical guidelines for the care and use of laboratory animals.Induction of Acute Kidney StressAcute renal ischemia was induced by bilateral renal artery occlusion. The right renal artery was immobilized, causing ischemia in the right kidney. This procedure was performed under general anesthesia using to ensure humane handling. In addition, rats in the experimental group received daily subcutaneous injections of adrenaline (dose [X mg/kg]) for [X days] to mimic prolonged activation of the sympathetic-adrenal system. The control group underwent the same surgical procedure but did not receive adrenaline injections.Blood samples were collected at multiple time points: Day 1, Day 3, Day 7, Day 10, and Day 14.Blood Sampling and Protein AnalysisBlood samples were obtained, and serum was separated by centrifugation at [rpm and time]. The total protein levels, as well as the protein fractions (albumin, globulins), were quantified using an automatic biochemical analyzer according to the manufacturer's instructions. The analyzer provided precise measurements of protein concentrations and fraction distributions.Hormonal AnalysisSerum levels of cortisol and adrenaline were measured using commercially available enzyme-linked immunosorbent assay (ELISA) kits. The sensitivity and specificity of the assays were validated according to the manufacturer’s instructions. Hormonal levels were measured at each time point to assess the activation of the sympathetic-adrenal system and hypothalamic-pituitary-adrenal (HPA) axis in response to acute renal ischemia.Statistical AnalysisData were expressed as mean ± standard deviation (SD). Differences between the experimental and control groups at each time point were analyzed using [statistical tests used, e.g., Student’s t-test, ANOVA]. Statistical significance was defined as p < 0.05. All analyses were performed using [software used, e.g., SPSS, GraphPad Prism].
4. Results and Discussion
- At the end of the first day, after modeling renal ischemia, an 18% decrease in total protein levels was observed compared to the intact group (from 6.1 g/dl to 5.0 g/dl), as well as a 30% reduction in albumin levels (from 3.8 g/dl to 2.65 g/dl). These changes are typical expressions of acute catabolic stress, in which stress hormones such as cortisol and adrenaline are activated, stimulating proteolysis (the breakdown of proteins) in skeletal muscles and other tissues. Furthermore, liver synthetic function decreases, especially in terms of albumin production. This condition leads to the diversion of liver resources toward the synthesis of acute-phase proteins. Additionally, the systemic inflammatory response is accompanied by the release of pro-inflammatory cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α). These mediators increase vascular permeability, leading to the leakage of proteins from blood vessels into the interstitial space, resulting in hypoproteinemia.The maximum decrease in total protein was observed on day 7, when its concentration reached 4.22 g/dl-a 31% reduction from the initial level, indicating ongoing metabolic and inflammatory stress.α1-globulins increased by 50% on day 7 (from 0.1 g/dl to 0.27 g/dl), which is characteristic of the acute inflammatory phase. This fraction includes α1-antitrypsin, α1-acid glycoprotein, and other acute-phase proteins, whose synthesis is activated by the liver in response to cytokines. The increase in this fraction indicates the activation of the systemic inflammatory response.α2-globulins showed an increase from days 1–3 (from 0.7 g/dl to 1.15 g/dl), which is also consistent with the typical model of the acute-phase response. However, by day 7, they sharply decreased (~50%) to 0.13 g/dl. This decrease may be due to ischemic liver damage, a decline in synthetic function, and reduced production of key proteins in this fraction, such as α2-macroglobulin, haptoglobin, and ceruloplasmin.β-globulins steadily increased from day 1 to day 7, reaching a maximum level of 2.18 g/dl (a 60% increase from the initial level). This may reflect the activation of the humoral elements of the immune system, including the complement system and transport proteins like transferrins.γ-globulins doubled on day 1 (from 0.9 g/dl to 1.88 g/dl, a 109% increase), and remained elevated through day 14 (2.01 g/dl on day 7, and 1.88 g/dl on day 14). This sustained increase indicates the prolonged activation of the adaptive immune response, including the synthesis of immunoglobulins. Possible causes of this include antigenic stimulation related to tissue damage and activation of immune cells, as well as the potential onset of autoimmune processes triggered by tissue destruction and systemic inflammation, without compromising immune tolerance (Figure 1).
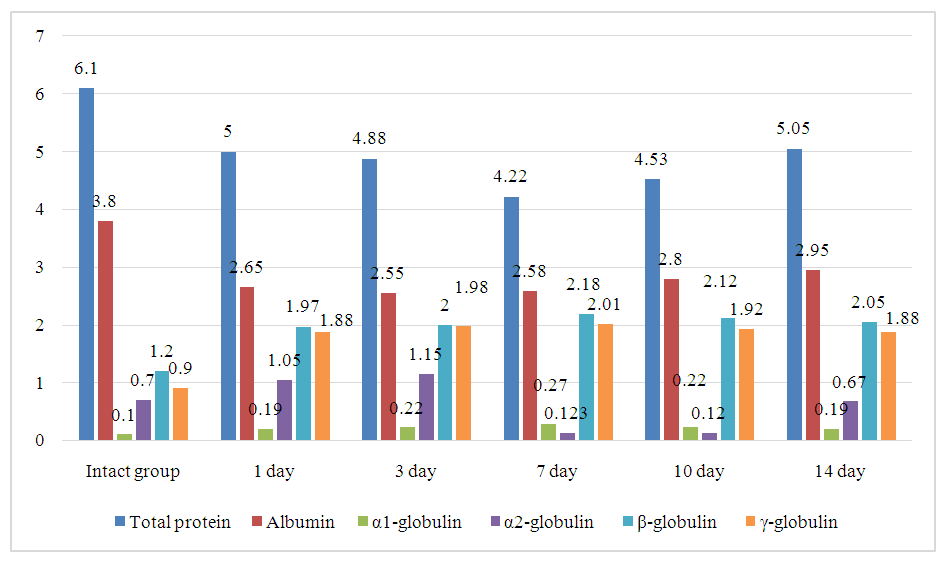 | Figure 1. Dynamics of Total Protein and Serum Protein Fractions in the Blood During Renal Ischemia in Laboratory Animals |
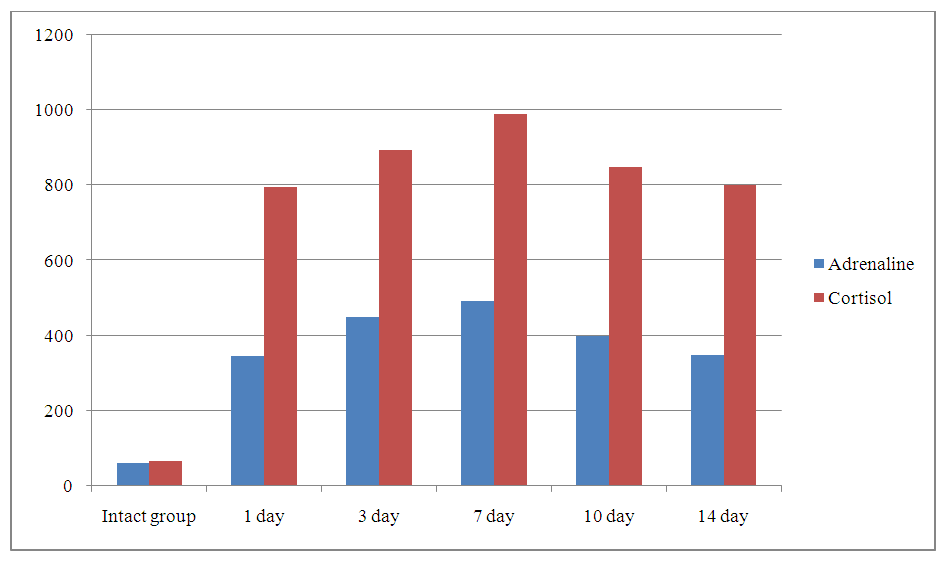 | Figure 2. Dynamics of Adrenaline and Cortisol in Blood Serum During Renal Failure in Laboratory Animals |
5. Conclusions
- In this study, we explored the systemic response to acute renal ischemia by analyzing changes in blood protein composition and hormonal profiles in a rat model. Our results demonstrate that acute renal ischemia induces significant alterations in serum protein levels, including a decrease in total protein and albumin, as well as changes in globulin fractions, indicative of a systemic inflammatory response. These changes are consistent with the activation of the acute-phase response, where the liver prioritizes the production of acute-phase proteins at the expense of albumin synthesis.Moreover, our findings reveal a pronounced activation of the sympathetic-adrenal system and the hypothalamic-pituitary-adrenal (HPA) axis, as evidenced by the marked increase in cortisol and adrenaline levels. The sustained elevation of these stress hormones suggests prolonged activation of the stress response, reflecting the organism's efforts to adapt to and cope with kidney dysfunction.The dynamic interaction between metabolic, hormonal, and inflammatory pathways highlights the complexity of the systemic response to acute renal ischemia. Our study emphasizes the importance of monitoring both protein and hormone levels as potential biomarkers for the progression of kidney injury. Additionally, these findings contribute to a deeper understanding of the physiological mechanisms underlying acute renal ischemia, which may inform the development of targeted therapeutic strategies for kidney-related disorders.Future studies should focus on further elucidating the molecular pathways involved in the acute-phase response and stress hormone regulation, as well as exploring potential interventions to mitigate the harmful effects of prolonged stress activation in the context of kidney injury.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML