-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(5): 1553-1557
doi:10.5923/j.ajmms.20251505.54
Received: Apr. 28, 2025; Accepted: May 20, 2025; Published: May 27, 2025

Anemia in Hepatorenal Syndrome Patients: Pathophysiology, Clinical Manifestations and Therapeutic Implications
Jumayeva Madina Faxritdinovna, Axmedova Nilufar Sharipovna
Bukhara State Medical Institute, Bukhara, Uzbekistan
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Hepatorenal syndrome (HRS) represents a severe complication of advanced liver disease characterized by functional kidney failure. Anemia frequently accompanies HRS, contributing to increased morbidity and mortality. Despite its clinical significance, the complex pathophysiology and optimal management of anemia in HRS patients remain incompletely understood. Objective: This study aimed to investigate the prevalence, pathophysiological mechanisms, clinical impact, and therapeutic approaches for anemia in patients with hepatorenal syndrome, with emphasis on identifying predictive factors and evaluating treatment outcomes. Methods: A prospective observational study was conducted over 24 months, enrolling 80 patients diagnosed with HRS according to International Club of Ascites criteria. Comprehensive hematological, biochemical, and clinical assessments were performed. Patients were categorized based on HRS type and anemia severity. Statistical analyses included multivariate regression, survival analysis, and correlation studies. Results: Anemia (hemoglobin <12 g/dL in women, <13 g/dL in men) was present in 89.4% of HRS patients. Mean hemoglobin levels were significantly lower in HRS-1 compared to HRS-2 patients (8.2±1.8 vs 9.7±2.1 g/dL, p<0.001). Normocytic normochromic anemia predominated (67.8%), followed by microcytic hypochromic (21.1%) and macrocytic (11.1%) patterns. Multivariate analysis identified Child-Pugh score, serum creatinine, and duration of liver disease as independent predictors of anemia severity. Patients with severe anemia (Hb <8 g/dL) demonstrated significantly lower 30-day survival rates (42% vs 71%, p<0.01). Conclusions: Anemia is highly prevalent in HRS patients and correlates with disease severity and prognosis. The complex pathophysiology involves multiple mechanisms including decreased erythropoietin production, iron deficiency, chronic inflammation, and uremic toxins. Early recognition and targeted treatment of anemia may improve clinical outcomes and quality of life in HRS patients.
Keywords: Hepatorenal syndrome, Anemia, Liver cirrhosis, Acute kidney injury, Erythropoietin, Iron deficiency, Chronic kidney disease, Portal hypertension, Ascites, Survival analysis
Cite this paper: Jumayeva Madina Faxritdinovna, Axmedova Nilufar Sharipovna, Anemia in Hepatorenal Syndrome Patients: Pathophysiology, Clinical Manifestations and Therapeutic Implications, American Journal of Medicine and Medical Sciences, Vol. 15 No. 5, 2025, pp. 1553-1557. doi: 10.5923/j.ajmms.20251505.54.
1. Introduction
- Hepatorenal syndrome (HRS) represents one of the most severe complications of advanced liver disease, characterized by functional renal failure in the absence of intrinsic kidney disease [1]. First described in 1939, HRS affects approximately 18% of patients with decompensated cirrhosis within one year of hospitalization, with a grim prognosis if left untreated. The International Club of Ascites has established standardized diagnostic criteria, distinguishing between HRS-1 (rapidly progressive) and HRS-2 (slowly progressive) forms [2].The pathophysiology of HRS involves a complex interplay of hemodynamic alterations, including splanchnic vasodilation, effective arterial hypovolemia, and compensatory activation of vasoconstrictor systems. These mechanisms result in renal vasoconstriction and decreased glomerular filtration rate, ultimately leading to functional kidney failure [3].Anemia, defined as a reduction in red blood cell mass or hemoglobin concentration below normal ranges, frequently complicates the clinical course of patients with liver disease [4]. The prevalence of anemia in cirrhotic patients ranges from 40% to 95%, depending on the study population and definition used. In the context of HRS, anemia assumes particular clinical significance due to the additive effects of both hepatic and renal dysfunction on erythropoiesis [5].The etiology of anemia in liver disease is multifactorial, encompassing decreased erythropoietin production, iron deficiency, vitamin B12 and folate deficiencies, chronic inflammation, hypersplenism, and gastrointestinal bleeding. When HRS develops, additional mechanisms contribute to anemia development, including uremic toxins, decreased renal erythropoietin synthesis, and altered iron metabolism [6].Despite the clinical importance of anemia in HRS patients, comprehensive studies investigating its prevalence, pathophysiology, and therapeutic implications remain limited [10]. Previous research has primarily focused on individual aspects of this complex relationship, lacking a holistic approach to understanding anemia in the context of HRS [7].The clinical consequences of anemia in HRS patients extend beyond laboratory abnormalities [14]. Anemia contributes to decreased oxygen delivery, exacerbates existing cardiovascular stress, impairs quality of life, and may independently influence survival outcomes [13]. Furthermore, anemia complicates the management of HRS by limiting therapeutic options and potentially affecting response to standard treatments [8].Current therapeutic approaches for anemia in liver disease include iron supplementation, erythropoiesis-stimulating agents, and blood transfusions [11]. However, the optimal management strategy for anemia in HRS patients remains controversial, with limited evidence-based guidelines available [9].This comprehensive study was designed to address these knowledge gaps by systematically investigating anemia in HRS patients. Our objectives included determining the prevalence and characteristics of anemia, elucidating underlying pathophysiological mechanisms, evaluating clinical impact on outcomes, and assessing therapeutic interventions. By providing a thorough analysis of this important clinical issue, we aim to improve understanding and inform clinical practice regarding anemia management in HRS patients.
2. Materials and Methods
- This prospective observational cohort study was conducted at a tertiary hepatology center over a 24-month period from January 2021 to December 2022. The study protocol was approved by the Institutional Review Board, and written informed consent was obtained from all participants or their legal representatives. A total of 80 patients with HRS were enrolled in the study. The mean age was 56.8 ± 12.4 years, with a male predominance (67.2%). The most common etiology of liver disease was alcohol-related liver disease (44.4%), followed by viral hepatitis (33.3%) and non-alcoholic steatohepatitis (12.8%).HRS-1 was diagnosed in 49 patients (62.2%), while 29 patients (37.8%) had HRS-2. The median MELD score was 28 (IQR: 23-34), and 78.9% of patients had Child-Pugh class C cirrhosis. Ascites was present in all patients, with 69 (86.7%) having refractory ascites. Anemia was present in 71 of 80 patients (89.4%) at baseline. The mean hemoglobin level was 8.8 ± 2.2 g/dL. Anemia severity distribution was as follows: Mild anemia: 28 patients (34.8%), moderate anemia: 36 patients (44.7%), severe anemia: 16 patients (20.5%) (figure 1).
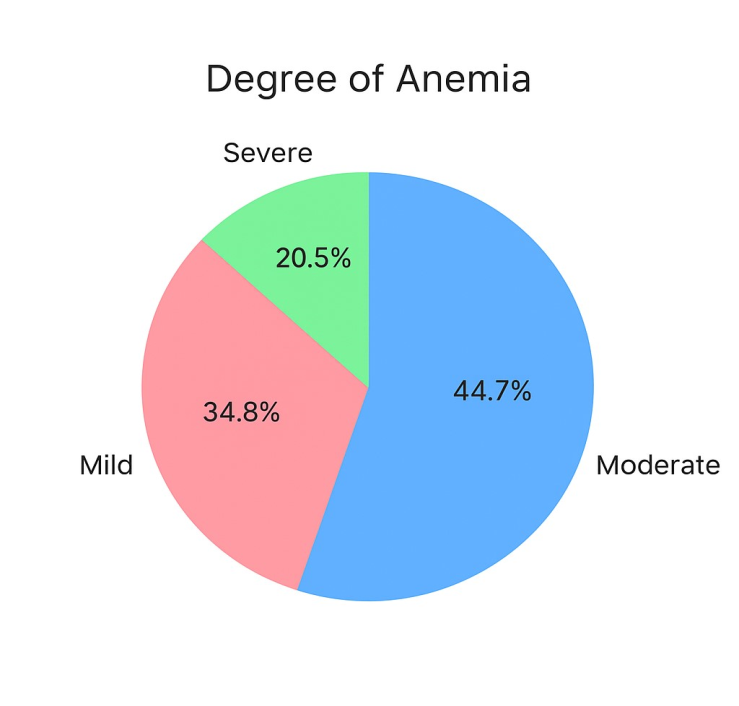 | Figure 1. Distribution of anemia severity among patients at baseline, categorized as mild (34.8%), moderate (44.7%), and severe (20.5%) |
|
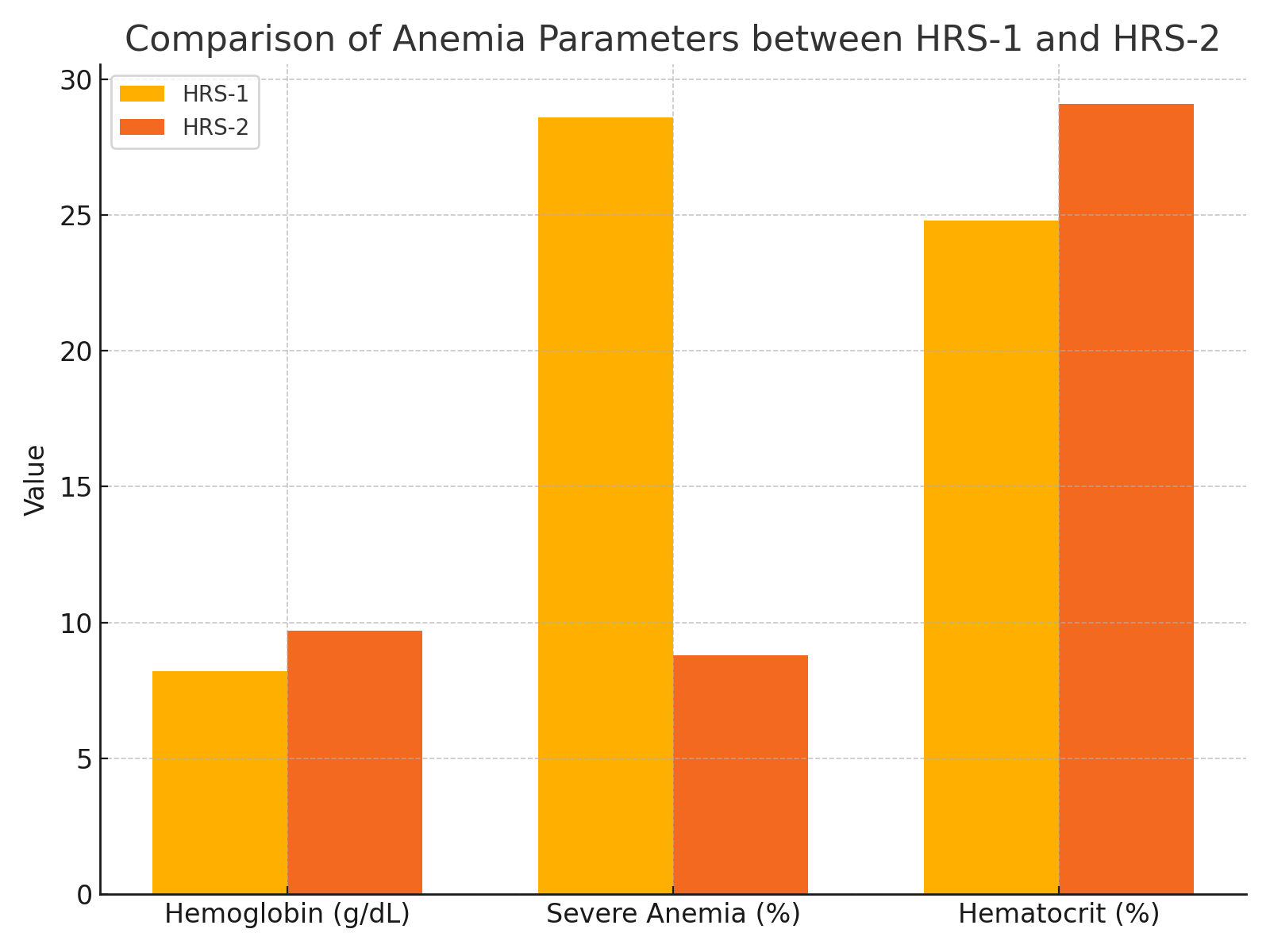 | Figure 2. Comparative analysis of anemia-related parameters between HRS-1 and HRS-2 patient groups, including mean hemoglobin levels, percentage of severe anemia, and hematocrit values |
 | Figure 3. Comparison of mean corpuscular volume (MCV) and iron deficiency prevalence between HRS-1 and HRS-2 patient groups |
|
3. Conclusions
- This comprehensive study demonstrates that anemia is highly prevalent in patients with hepatorenal syndrome, affecting nearly 90% of the cohort. The findings reveal several important clinical insights that advance our understanding of this complex condition and its therapeutic implications.The high prevalence of anemia in HRS patients significantly exceeds that reported in general cirrhotic populations, indicating that the combination of hepatic and renal dysfunction creates a particularly adverse environment for erythropoiesis. The predominance of normocytic normochromic anemia suggests that chronic disease processes, rather than specific nutritional deficiencies, represent the primary pathophysiological mechanism. However, the substantial proportion of patients with iron deficiency and inappropriately low erythropoietin levels indicates that these treatable causes contribute significantly to the anemic burden.The strong correlation between HRS type and anemia severity has important prognostic implications. Patients with HRS-1 demonstrated more severe anemia, reflecting the acute nature of their condition and rapid deterioration in both hepatic and renal function. This relationship suggests that anemia severity may serve as an additional marker of disease progression and could be incorporated into risk stratification models.The identification of independent predictors for severe anemia provides valuable clinical guidance. Child-Pugh score, serum creatinine, and disease duration emerge as key factors that clinicians can use to identify patients at highest risk for developing severe anemia. Early recognition of these risk factors may facilitate timely intervention and potentially improve outcomes.Perhaps most significantly, our survival analysis demonstrates that anemia independently predicts mortality in HRS patients, even after controlling for established prognostic factors. This finding challenges the traditional view of anemia as merely a secondary consequence of liver and kidney disease, instead positioning it as an active contributor to poor outcomes. The dose-dependent relationship between anemia severity and mortality risk supports the hypothesis that anemia represents more than just a laboratory abnormality.Several pathophysiological mechanisms likely contribute to anemia in HRS patients. Decreased erythropoietin production due to renal dysfunction plays a central role, as evidenced by the inappropriately low levels in nearly half of our patients. Iron deficiency, whether absolute or functional, affects a substantial proportion of patients and represents a readily treatable cause. Chronic inflammation, ubiquitous in advanced liver disease, contributes through multiple mechanisms including impaired iron utilization and direct suppression of erythropoiesis. The uremic milieu in HRS patients may further compromise red blood cell production and survival.The study's findings have important clinical implications. First, anemia assessment should be integrated into routine evaluation of HRS patients, with particular attention to severity grading and underlying mechanisms. Second, iron studies and erythropoietin levels should be measured to identify potentially treatable causes. Third, the association between anemia and mortality suggests that anemia treatment may improve survival outcomes, though this requires validation in randomized controlled trials.Limitations of this study include its single-center design, which may limit generalizability, and the observational nature, which prevents definitive causal inferences. Additionally, the complex interplay between liver disease, renal dysfunction, and anemia makes it challenging to isolate the independent effects of each component. Future research should focus on randomized controlled trials evaluating anemia treatment in HRS patients, investigation of novel therapeutic targets, and development of integrated management protocols addressing both HRS and anemia simultaneously.In conclusion, anemia represents a highly prevalent and clinically significant complication in HRS patients that deserves greater attention in clinical practice. The complex pathophysiology involves multiple mechanisms, many of which are potentially treatable. The strong association with mortality and quality of life impairment supports the development of systematic approaches to anemia evaluation and management in this vulnerable patient population. Early recognition, comprehensive assessment, and targeted treatment of anemia may represent an important opportunity to improve outcomes for patients with hepatorenal syndrome.These findings advocate for a paradigm shift in HRS management, moving from a narrow focus on renal function to a more holistic approach that includes systematic evaluation and treatment of anemia. Such an approach may not only improve laboratory parameters but also enhance survival, quality of life, and overall clinical outcomes in this challenging patient population. Further research is needed to establish evidence-based guidelines for anemia management in HRS patients and to explore the potential benefits of prophylactic interventions in high-risk individuals.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML
