-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(5): 1530-1535
doi:10.5923/j.ajmms.20251505.50
Received: May 2, 2025; Accepted: May 25, 2025; Published: May 27, 2025

Prognostic Significance of Micronutrient Deficiencies in the Progression of Chronic Kidney Disease (CKD) at Various Stages and to Improve Methods for Correcting These Deficiencies
Sulaymonova Gulnoza Tulkinjanovna
Bukhara State Medical Institute named after Abu Ali ibn Sina, Bukhara, Uzbekistan
Correspondence to: Sulaymonova Gulnoza Tulkinjanovna, Bukhara State Medical Institute named after Abu Ali ibn Sina, Bukhara, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Micronutrient deficiencies are increasingly recognized as crucial factors influencing the pathogenesis and progression of chronic kidney disease (CKD). This study investigates the prognostic role of essential micronutrient imbalances—including zinc, selenium, copper, magnesium, and vitamin D—across different stages of CKD. By analyzing clinical and biochemical markers in patients at early (stage 1–2), moderate (stage 3), and advanced (stage 4–5) stages of CKD, the study identifies correlations between deficiency severity and disease progression. Furthermore, the efficacy of targeted micronutrient correction strategies, such as oral supplementation and dietary interventions, is assessed. The findings highlight that timely detection and correction of micronutrient deficiencies can slow CKD progression, improve renal outcomes, and enhance patients’ quality of life. These results underscore the importance of integrating micronutrient evaluation into routine CKD management protocols.
Keywords: Chronic Kidney Disease (CKD), Micronutrient Deficiency, Disease Progression, Zinc, Selenium, Vitamin D, Renal Function Correction Strategies
Cite this paper: Sulaymonova Gulnoza Tulkinjanovna, Prognostic Significance of Micronutrient Deficiencies in the Progression of Chronic Kidney Disease (CKD) at Various Stages and to Improve Methods for Correcting These Deficiencies, American Journal of Medicine and Medical Sciences, Vol. 15 No. 5, 2025, pp. 1530-1535. doi: 10.5923/j.ajmms.20251505.50.
1. Introduction
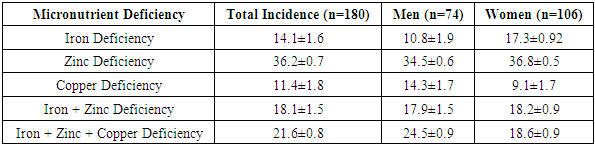
- Chronic kidney disease (CKD) is a collective term indicating kidney tissue damage regardless of the etiological causes. CKD is diagnosed when there is evidence of kidney damage for three months or more, indicated by markers such as albuminuria/proteinuria, pathological casts in urine, or morphological changes, or when the glomerular filtration rate (GFR) is less than 60 mL per minute per 1.73 m² body surface area [1,8].According to 1990 data, CKD ranked 27th as a cause of death, but by 2010, it had moved to 18th place. These figures confirm that over the last 20 years, the mortality due to severe complications of this disease has significantly increased among the population. Although there are no exact statistics on the prevalence of CKD in Uzbekistan, the widespread nature of diseases that lead to CKD suggests a high incidence of severe complications in the country.Kidney damage caused by various diseases is becoming an increasingly important issue globally. Comprehensive epidemiological studies on the prevalence, early diagnosis, treatment principles, and prevention measures of CKD have been conducted, and continue to this day. The first study in this regard was conducted in the USA in 2002 by the KDOQI (Kidney Disease Outcomes Quality Initiative) National Kidney Foundation, where a national CKD concept was developed. Based on this concept, two large-scale epidemiological studies were conducted in the USA, focusing on the prevalence of CKD, its complications, disability, and mortality rates, as well as the financial costs associated with CKD treatment. This included the NHANES (National Health and Nutrition Examination Survey) program from 2005-2010 and the KEEP (The Kidney Early Evaluation Program) from 2000–2011. Research between 2003 and 2018 showed that 14.8% of the population in the USA had CKD.In 2005, epidemiological studies on CKD prevalence were conducted in the Netherlands (PREVEND), Spain (EPIRCE), Japan (Imai E et al., 2011), Russia (2012), India (SEEK-India, 2013), Kazakhstan and Tajikistan (2012-2017), which also included national programs for the early detection and prevention of CKD. Results showed that approximately 10% of the global population suffers from CKD, with variations: 7% in South Asia, 8% in Africa, 11% in North America, and 12% in Europe, East Asia, and Latin America.Some conclusions from research suggest that 10% of the world’s population has CKD, which is similar in number to the global count of individuals with diabetes [2,5]. The prevalence of CKD increases with age, reaching 20% after 60 years and 35% after 70 years [3,4].Studies indicate that among individuals with hypertension, CKD prevalence is 36.6% [1,6]. Kidney changes significantly increase the risk of cardiac death, doubling or even tripling the incidence of such deaths [5,9]. Unfortunately, kidney damage in hypertensive individuals is often not detected until advanced stages, after years of progression, when chronic kidney failure manifests [7,8].The mechanisms of kidney damage in hypertension are well-documented, though recent years have introduced new perspectives on this process. A review of literature confirms that nephropathy progression is complex, involving multiple stages with disturbances in renal hemodynamics and the initiation of various pathological processes.For many years, clinical practice has relied on markers like blood creatinine levels, GFR (glomerular filtration rate), and microalbuminuria/proteinuria for diagnosing kidney damage. However, these markers have several limitations [4,8]. Creatinine has been used for almost 50 years and remains a reliable marker for clinical practice. However, its levels can vary based on a person’s age, gender, metabolic state, muscle mass, and electrolyte balance [2,8]. Additionally, determining GFR through formulas like Cockcroft-Gault, MDRD, and CKD-EPI has become common practice, although the first two formulas have certain limitations. In recent years, the CKD-EPI formula is more widely used in practice [1,14], but even this method relies on creatinine measurements, which still carry inherent limitations.Both chronic kidney disease and micronutrient deficiencies are among the most widespread and debilitating conditions. With an increasing average life expectancy, the global issue of micronutrient deficiencies (micronutrient malnutrition) has been acknowledged as a serious concern. The deficiency of micronutrients poses significant public health issues, as inadequate nutrition impacts physical and mental development, leading to delayed childhood development, increased susceptibility to various diseases, and reduced work capacity [2,5].Recent studies have shown that the deficiency of essential micronutrients impacts multiple organ functions, including the kidneys, and can cause serious pathological changes [3,7]. In particular, micronutrient deficiencies like zinc and iron lead to disturbances in reproductive health, especially in women of fertile age, which may result in ovarian dysfunction [1,5,8].In chronic kidney disease’s terminal stages, there is limited data on micronutrient imbalances, especially at stages II-III and the pre-dialysis period. Kidney dysfunction, which plays a key role in maintaining homeostasis, impacts the micronutrient status and contributes to the worsening of the disease and complications in cardiovascular, digestive, and other systems. Early identification and correction of micronutrient deficiencies are essential, especially in high-risk patients, to improve health outcomes [5,9,12].Recent studies have also highlighted the importance of understanding the biogeochemical factors contributing to micronutrient imbalances. These include environmental, anthropogenic, and climatic factors that continuously affect the human body [11,13].For example, studies by E.G. Kuznetsova and co-authors (2017, Russia) have shown the role of various micronutrients, including magnesium, cobalt, zinc, and selenium, in the development of pyelonephritis and dismetabolic nephropathy in children.A study by Marcello Tonelli et al. (2020, Canada) on patients undergoing hemodialysis showed that deficiencies in zinc and selenium correlated with worse post-dialysis outcomes.Iron, zinc, copper, iodine, selenium, and manganese are among the essential micronutrients whose deficiency is closely related to various pathological conditions. Scientific research continues to explore the pathophysiological importance of micronutrient deficiencies across various medical fields [15].In particular, A.M. Shilov (2008) demonstrated the significance of magnesium deficiency in cardiovascular and cerebral pathologies. Magnesium deficiency was found to be prominent in the terminal stages of CKD.Research by D. Yonova et al. (2012) highlighted the role of micronutrient deficiencies, such as zinc, in dialysis patients, with clinical symptoms including altered taste perception and poor appetite, as well as gastrointestinal disturbances.Iron deficiency is particularly important in the anemia associated with CKD. Anemia in CKD is closely linked to impaired iron absorption, gastrointestinal bleeding, or excessive erythropoietin therapy during treatment.The optimal amount of micronutrients is essential for normal organ function, immune response, cell proliferation, bone and muscle development, and overall homeostasis.Study objective: To determine the prognostic significance of micronutrient deficiency in the progression of chronic kidney disease and to improve methods for its correction.
2. Materials and Methods
- The study involved patients from Bukhara City Central Polyclinic, who were diagnosed with nephropathy and received inpatient treatment in 2022 and 2024. Clinical, instrumental, and laboratory analyses were performed, and 124 patients were prospectively selected. The diagnosis of nephropathy was made based on the criteria established by the American Society of Nephrology in 2010. Exclusion criteria included co-existing kidney diseases, stage II-III hypertension, diabetes for more than 10 years, and history of myocardial infarction or stroke.The progression of the main disease, comorbidities, and the pharmacotherapy administered to the patients were assessed. Clinical blood tests, urine tests (Nechiporenko test and daily protein loss analysis), and renal ultrasound were performed. Kidney function was assessed using the MDRD formula based on serum creatinine and urea levels. Initial data were obtained upon admission to the hospital.Patients with hypertension stages II-III, decompensated diabetes, and nephropathy stages 4 and 5 were excluded from the study. Anti-hypertensive and nephroprotective drugs were administered alongside micronutrient bioactive supplements.Laboratory tests included hemoglobin, general urine analysis, daily protein loss, serum creatinine, urea, albumin, ALT, and lipid profile. Serum creatinine was analyzed using an enzymatic method. The main micronutrients (Fe, Zn, Cu, Se) were measured before and after treatment.
3. Research Results
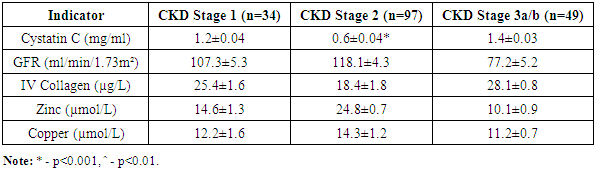
- According to the degree of nephropathy detected in the selected patient groups, i.e., based on laboratory markers such as cystatin C, nephrinuria, 24-hour PU/AU, and tests evaluating renal tubular function, angiotensin II receptor blockers were used as nephroprotective drugs according to the blood pressure level. After treatment, renal function tests (KFT) and the glomerular filtration rate (GFR) were assessed based on serum creatinine and cystatin C levels at 3 and 6 months. The GFR was used as a prognostic indicator for renal recovery.Furthermore, to assess the renal glomerular and tubular system after treatment, modern markers of nephropathy (nephrinuria, VEGF A, TGF ß1, and IV collagen) were determined. Comparative analysis of the results before and after treatment was performed, and the efficacy of the drug was studied based on the impact on laboratory and instrumental markers, leading to the development of an algorithm and principles for treatment based on the duration of the disease and the levels of diagnostic markers.Figures 1-3 present a comparative analysis of traditional and modern markers of nephropathy diagnosis before and after treatment in patients with various etiologies.
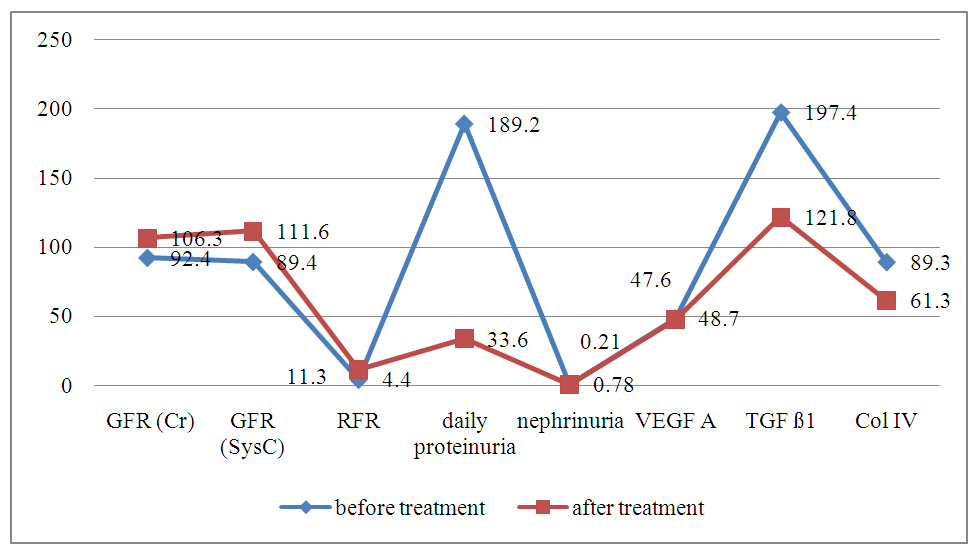 | Figure 1. Comparative analysis of markers for diagnosing nephropathy in the early stages of hypertension and high blood pressure |
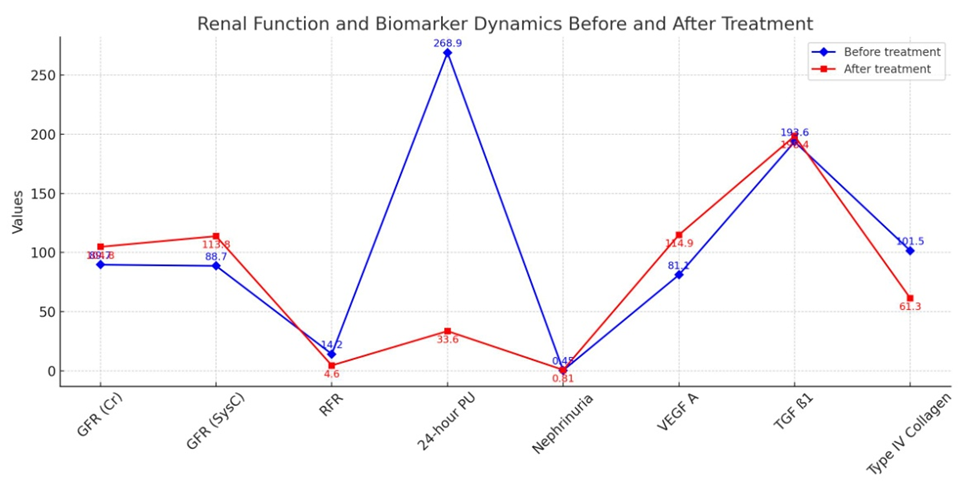 | Figure 2. Comparative analysis of nephropathy diagnostic markers during the compensation phase of Type II diabetes |
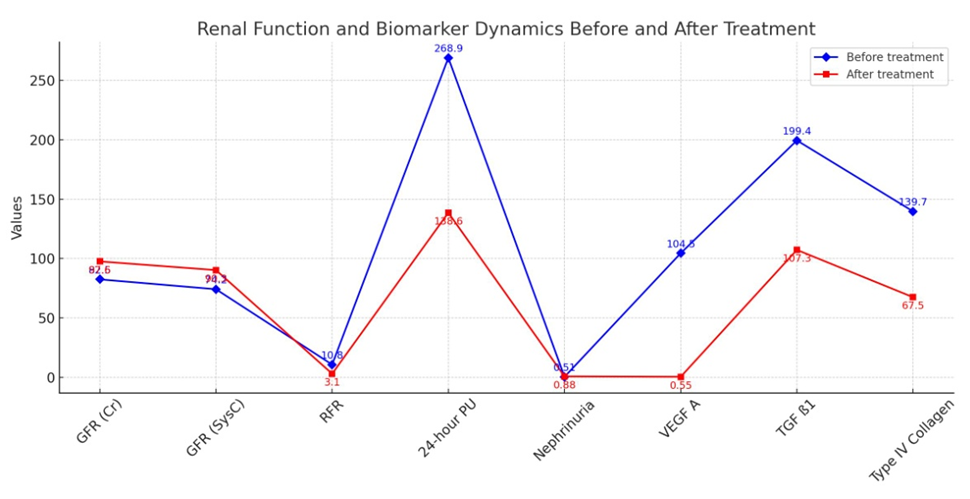 | Figure 3. Comparative analysis of nephropathy diagnostic markers in the early stages of chronic glomerulonephritis |
|
|
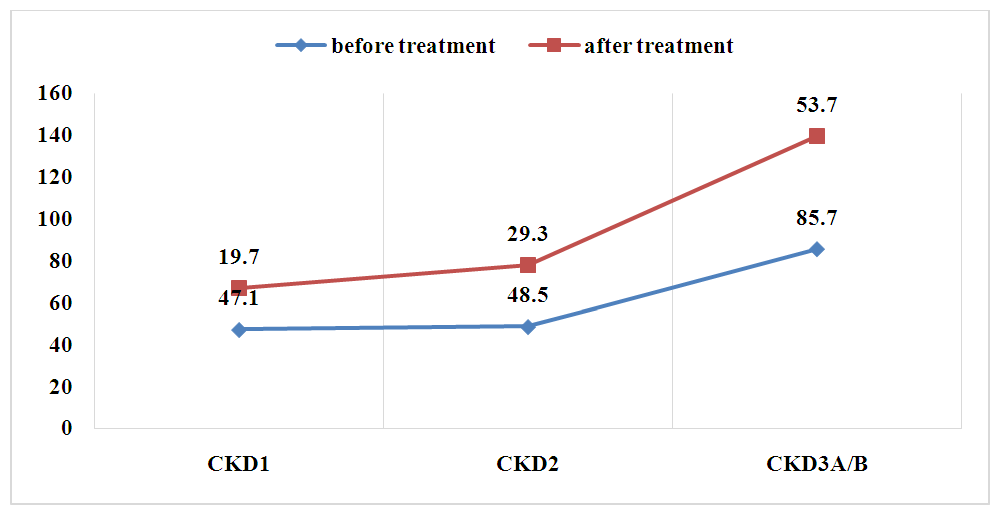 | Figure 4. Comparative analysis of anemia frequency before and after treatment in various stages of CKD |
4. Conclusions
- A comparison of ferritin levels before and after treatment showed a slight but not statistically significant decrease (p=0.05). This further emphasizes the potential role of zinc in alleviating anemia, particularly in patients with polydeficient and chronic inflammatory anemia in CKD.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML