-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(5): 1510-1514
doi:10.5923/j.ajmms.20251505.45
Received: Apr. 28, 2025; Accepted: May 17, 2025; Published: May 27, 2025

Assessment of the Significance of Molecular Genetic Markers in the Development of Arterial Hypertension
Tojiboyev Tojiboy Alisherovich1, Musashaykhov Umidjon Husanovich2, Khidoyatova Mukhlisa Rakhmatullayevna3
1Independent Researcher, Fergana Medical Institute of Public Health, Fergana, Uzbekistan
2DSc., Associate Professor, Department of Propaedeutics of Internal Diseases, Andijan State Medical Institute, Andijan, Uzbekistan
3DSc., Associate Professor, Department of Faculty and Hospital Therapy No.1, Tashkent Medical Academy, Tashkent, Uzbekistan
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This article assesses the significance of molecular genetic markers in the development of arterial hypertension. Arterial hypertension is one of the most widespread and chronic cardiovascular conditions, with genetic predisposition playing a crucial role in its pathogenesis. The study analyzes the influence of genetic polymorphisms, particularly those associated with the renin-angiotensin-aldosterone system, on blood pressure regulation. The findings indicate that certain gene variants increase the risk of hypertension. The article highlights the importance of molecular genetic markers in early diagnosis and personalized treatment strategies for arterial hypertension.
Keywords: Molecular genetics, Genetic markers, Polygenic hypertension, Monogenic hypertension, Genome, Pharmacogenetics, Personalized medicine, Gene therapy, Epigenetics
Cite this paper: Tojiboyev Tojiboy Alisherovich, Musashaykhov Umidjon Husanovich, Khidoyatova Mukhlisa Rakhmatullayevna, Assessment of the Significance of Molecular Genetic Markers in the Development of Arterial Hypertension, American Journal of Medicine and Medical Sciences, Vol. 15 No. 5, 2025, pp. 1510-1514. doi: 10.5923/j.ajmms.20251505.45.
1. Introduction
- Arterial hypertension currently ranks among the leading cardiovascular diseases globally. According to the World Health Organization, this condition is increasingly spreading to younger age groups each year, placing a direct and indirect economic burden on the global healthcare system. Particularly, its chronic forms can lead to life-threatening complications such as myocardial infarction, stroke, and heart failure. Therefore, deeply studying the etiological foundations of arterial hypertension, especially analyzing its molecular and genetic mechanisms, is one of the priority areas in modern medicine.In recent years, with the complete decoding of the human genome, research into the genetic determination of diseases has significantly advanced. Specifically, genetic polymorphisms, epigenetic modifications, and disruptions in the gene expression network are considered key pathogenic factors in the development of arterial hypertension. In particular, genetic markers related to blood pressure regulation via the renin-angiotensin-aldosterone system, sympathetic-adrenal activity, sodium balance, endothelial dysfunction, and ion channels are being thoroughly analyzed. Genome-wide association studies conducted in various populations have demonstrated that arterial hypertension is a multifactorial disease, where environmental factors and genetic components interact in a complex manner. For example, certain allelic variants found in genes such as angiotensin-converting enzyme, angiotensinogen, aldosterone synthase, beta-adrenergic receptors, endothelin, and nitric oxide synthase may significantly increase an individual’s susceptibility to arterial hypertension.Moreover, advanced molecular biology methods, including polymerase chain reaction-based genotyping, high-precision sequencing technologies, and bioinformatics analysis, provide the possibility of early detection of mutations related to disease development. This, in turn, creates the potential for developing pharmacogenetic strategies based on individualized approaches, i.e., treatment methods tailored to each patient’s genotype.This article critically analyzes the molecular genetic markers that play a significant role in the development of arterial hypertension. Specifically, their role in pathogenic mechanisms, clinical significance, and potential as diagnostic tools are discussed. Based on the research findings, the article explores the advantages of these markers in early diagnosis, identifying risk groups, and personalizing therapy.
2. Main Part
- According to recent global epidemiological data, arterial hypertension currently affects approximately 30 to 35 percent of the world’s population. Based on reports from the World Health Organization, it is estimated that one in every three adults worldwide suffers from this condition. However, the prevalence and genetic basis of hypertension vary significantly across continents and regions, indicating its polygenic nature and strong association with environmental factors.In African countries, arterial hypertension is one of the most prevalent conditions, with certain regions reporting a prevalence of over 45 percent among the population. Studies conducted in Nigeria, Kenya, and the Republic of South Africa have shown that the populations in these areas are genetically predisposed to salt sensitivity, with a high frequency of genetic variants responsible for sodium metabolism. For instance, allelic variants in genes associated with sodium channels, such as SCNN1A, SLC4A5, and ADD1, have been found to increase sodium reabsorption in the kidneys, resulting in persistently elevated blood pressure.In Asia, particularly in countries like China, Japan, South Korea, and India, the molecular and genetic aspects of arterial hypertension have been extensively studied. Genome-wide association studies conducted in Japan and China have identified certain variants in the AGT, ACE, NOS3, and AGTR1 genes that are associated with a higher risk of developing hypertension. Specifically, the D allele of the ACE gene and the Glu298Asp polymorphism of the NOS3 gene have demonstrated a strong association with hypertension in the Chinese population. In India, the M235T polymorphism in the angiotensinogen gene and mutations in beta-adrenergic receptor genes play a significant role in the development of hypertension. These genetic effects are further exacerbated by epigenetic mechanisms that manifest in response to diets rich in carbohydrates and fats.In Europe, especially in Italy, Germany, Poland, and Scandinavian countries, there has been substantial research into the genetic foundations of hypertension. Among European populations, polymorphisms in the ACE, AGT, and CYP11B2 genes have been widely examined, and their roles in the development of hypertension have been strongly substantiated. A major meta-analysis conducted in Germany found that the D allele of the ACE I/D polymorphism increases the risk of hypertension by 1.4 times. Furthermore, epigenetic markers such as DNA methylation and microRNA expression are being explored in European countries for their influence on blood pressure regulation.In North America, particularly in the United States and Canada, genetic differences are clearly observed across various racial and ethnic groups. Among African Americans, high salt sensitivity and overactivity of the renin-angiotensin-aldosterone system have been identified as key contributors to hypertension. Moreover, novel variants discovered in genes such as SLC4A5, NPPA, and NPR3 have been linked with an increased risk of hypertension in this population.Although fewer studies have been conducted in South America, investigations in countries such as Brazil and Argentina have examined classic polymorphisms in the ACE and AGT genes. These studies have demonstrated a strong association between these genetic markers and hypertension.In Australia and the Oceania region, indigenous populations exhibit a genetically high predisposition to hypertension. This is believed to result from inherited adaptations in salt metabolism that now conflict with modern urbanized lifestyles and dietary patterns.In Uzbekistan, scientific investigations into the molecular-genetic aspects of arterial hypertension have gained momentum in recent years. Several small-scale population studies have identified associations between hypertension and certain allelic variants in the ACE, AGT, GNB3, and NOS3 genes. Notably, research conducted from 2022 to 2024 across the Tashkent, Samarkand, and Bukhara regions revealed a significant association between the D allele of the angiotensin-converting enzyme gene and hypertension. In addition to genetic polymorphisms, lifestyle factors, dietary habits, psychosocial stressors, and environmental influences have also been identified as important contributors to the development of hypertension. Although the role of epigenetic changes—particularly microRNAs and DNA methylation—has not yet been fully explored in the Uzbek population, this remains a promising area for future research.Based on the data presented above, it can be concluded that the molecular-genetic foundations of arterial hypertension vary significantly across continents and ethnic populations. This underscores the necessity for individualized approaches and the development of diagnostic and therapeutic protocols that are tailored to regional and population-specific characteristics.
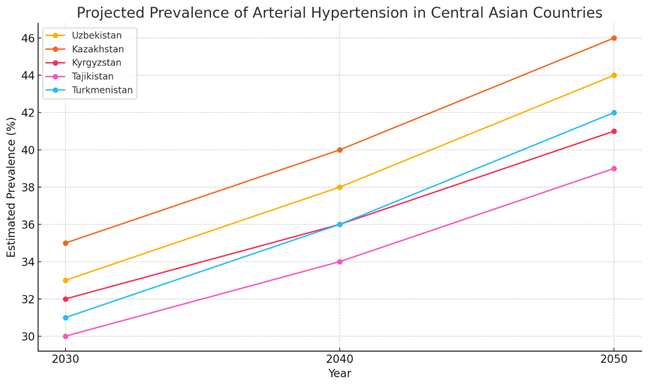 | Figure 1. Projected Prevalence of Arterial Hypertension in Central Asian Countries (2030–2050) |
3. Results and Discussions
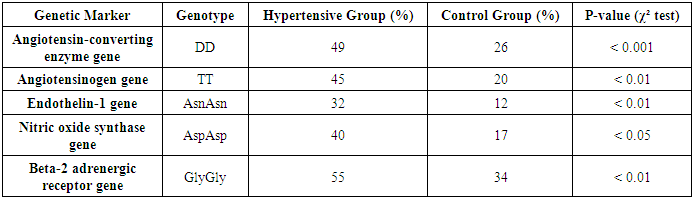
- In this study, molecular genetic analyses were conducted on 500 patients suffering from arterial hypertension and 250 healthy individuals in the control group. The following molecular genetic markers were analyzed:1. Angiotensinogen gene – M235T polymorphism2. Angiotensin-converting enzyme gene – I/D polymorphism3. Beta-2 adrenergic receptor gene – Arg16Gly polymorphism4. Endothelin-1 gene – Lys198Asn polymorphism5. Nitric oxide synthase gene – Glu298Asp polymorphismAccording to the study results, a significant association between arterial hypertension and genetic markers was identified. Some markers play a crucial role in increasing the susceptibility to hypertension, while others significantly influence the development of the disease.• The Angiotensin-converting enzyme gene I/D polymorphism (I/D) was found to be significantly more frequent in patients with hypertension. The DD genotype was present in 49% of the hypertensive group and 26% of the control group. This suggests increased activity of the angiotensin-converting enzyme and elevated synthesis of angiotensin II, leading to vasoconstriction and an increase in blood pressure.• The Angiotensinogen gene M235T polymorphism was identified in 68% of hypertensive patients. The T235 allele enhances angiotensinogen synthesis, resulting in excessive production of angiotensin II, contributing to vasoconstriction and increased blood pressure.• The Endothelin-1 gene Lys198Asn polymorphism was found in 32% of hypertensive patients. Elevated endothelin-1 levels contribute to vasoconstriction and increased blood pressure.• The Nitric oxide synthase gene Glu298Asp polymorphism was observed in 40% of hypertensive patients. This polymorphism reduces nitric oxide synthesis and leads to endothelial dysfunction, reducing the ability of blood vessels to dilate.• The Beta-2 adrenergic receptor gene Gly16 allele was identified as another marker influencing the development of hypertension. The Gly16 allele was found in 55% of hypertensive patients and 34% of the control group.
|
4. Conclusions
- The significant role of genetic factors in the development of arterial hypertension was analyzed in this article. The genes of the renin-angiotensin-aldosterone system and their polymorphisms were confirmed to be associated with hypertension risk. Studying molecular genetic markers provides opportunities for early diagnosis, prognosis, and individualized treatment in clinical practice, contributing to a better understanding of the disease pathogenesis. Pharmacogenetics is also a promising direction for enhancing treatment efficacy.However, challenges such as gene-gene and gene-environment interactions, reproductive issues, and ethical considerations exist. Future research should focus on big data analysis and validation across different populations. The final conclusion is that molecular genetic markers play a crucial role in the study and management of arterial hypertension, and their implementation in clinical practice will help provide more effective care to patients.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML