-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(5): 1487-1490
doi:10.5923/j.ajmms.20251505.40
Received: Apr. 29, 2025; Accepted: May 17, 2025; Published: May 27, 2025

Morphological Changes in the Lungs of 5-Month-Old Albino Rats Following Chronic Kidney Disease
Avezova Dilora Botirovna
Department of Anatomy, Clinical Anatomy (OHTA), Bukhara State Medical Institute named after Abu Ali Ibn Sino, Bukhara, Uzbekistan
Correspondence to: Avezova Dilora Botirovna, Department of Anatomy, Clinical Anatomy (OHTA), Bukhara State Medical Institute named after Abu Ali Ibn Sino, Bukhara, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This scientific study investigates the histomorphological changes occurring in lung tissues under conditions of chronic kidney disease (CKD). The experiment involved 30 five-month-old albino rats. CKD was induced using a modified version of the Greven method by intramuscular injection of a 5% glycerol solution. Histological sections of the lung revealed pathological alterations such as thickening of the alveolar septa, interstitial fibrosis, alveolar collapse, and inflammatory infiltration. The obtained results demonstrated that severe morphological disturbances develop in lung tissue against the background of CKD.
Keywords: Chronic kidney disease, Lung morphology, Histology, Rat, Glycerol, Greven method, Experimental model
Cite this paper: Avezova Dilora Botirovna, Morphological Changes in the Lungs of 5-Month-Old Albino Rats Following Chronic Kidney Disease, American Journal of Medicine and Medical Sciences, Vol. 15 No. 5, 2025, pp. 1487-1490. doi: 10.5923/j.ajmms.20251505.40.
1. Introduction
- Chronic kidney disease (CKD) is a complex syndrome characterized by the progressive decline of renal function, which leads to pathological changes in various organ systems including the cardiovascular, endocrine, and respiratory systems. According to the World Health Organization, the global prevalence of CKD is steadily increasing, significantly affecting patients’ quality of life and life expectancy [1].Due to the close physiological relationship between the kidneys and lungs, impaired renal function can cause a range of respiratory system disorders. In CKD, hemodynamic changes, accumulation of nitrogenous waste products, hypoxemia, and disturbances in acid-base balance can lead to morphological alterations in lung tissues. Additionally, prolonged exposure to uremic toxins may damage alveolar barriers, capillaries, and bronchiolar structures [2,3,4].While the impact of CKD on organs such as the heart, liver, and brain has been extensively studied, data on lung tissue changes remain limited. In particular, there is a lack of research focusing on young experimental animal models such as 5-month-old white outbred rats which highlights the relevance and urgency of this topic [5,8].In this study, morphological changes in the lungs were histologically examined in rats with CKD induced using the Greven method. The aim was to identify and characterize structural alterations in lung tissues resulting from CKD and to evaluate age-related differences in these pathological changes [6,7,9,10].Chronic kidney disease (CKD) is a serious medical, social, and economic problem. It is characterized by the steady increase in the number of affected patients, the high cost of treatment, and poor work-related prognosis [12]. Chronic kidney disease is widespread globally, affecting more than 10% of the population, which corresponds to over 800 million individuals. This condition poses a significant public health burden, particularly in low- and middle-income countries, and is among the leading causes of death [13].CKD and its consequences are among the few non-communicable diseases marked by a rising mortality rate over the past two decades [13]. The increasing prevalence of CKD is associated with longer life expectancy and reduced early mortality from complications of hypertension and diabetes. In addition, advancements in treatment such as the introduction of dialysis have dramatically changed the prognosis for CKD patients. However, there remains a growing need for more information about the condition of internal organs and systemic changes during prolonged uremia [14].Chronic kidney disease leads to distinct structural changes in the interstitial tissues of the lungs. Microcirculatory disturbances occur along with redistribution of the intercellular matrix in the connective tissue [14,15]. In the bronchopulmonary system, mixed-type ventilatory impairments and reduced pulmonary diffusing capacity are observed [16]. One of these manifestations is pulmonary calcification, which contributes to decreased lung volume and impaired gas exchange [17]. Studies have shown that the lungs of uremic rats exhibit changes such as massive atelectasis, chronic edema, bronchiolitis, and pulmonary fibrosis. Lung damage during renal failure follows a progressive course [18].Pulmonary hypertension is also a common pathological condition associated with CKD and end-stage renal disease (ESRD). This condition not only leads to adverse clinical outcomes but also increases the risk of mortality. Although the relationship between pulmonary hypertension and mortality is not yet fully understood, observational studies support this association [19]. The interaction between the lungs and kidneys is clinically complex and may lead to fluid and acid-base imbalance, vascular tone changes, and hemodynamic disorders in the lungs. The kidneys influence overall physiology through sodium and water retention, as well as by disrupting perfusion and filtration processes [20].Other systemic manifestations of CKD include malnutrition, muscle atrophy, anemia, osteoporosis, and cardiovascular diseases. These conditions highlight the crucial role of endothelial dysfunction in the early stages of CKD [11]. Moreover, increased venous pressure may disrupt the alveolar-capillary interface and lead to damage of capillary walls [12].In this context, the main objective of the present study is to investigate morphological changes occurring in lung tissue under experimental chronic kidney disease (CKD) conditions, with a specific focus on the use of the Greven method. The findings of this research will contribute to a better understanding of lung tissue alterations associated with CKD and may serve as a foundation for developing future therapeutic strategies for pulmonary pathologies.
2. Materials and Methods
- The study involved 30 healthy, 5-month-old outbred white rats weighing between 180–220 grams. To experimentally induce chronic kidney disease (CKD), a modified version of the Greven method was used. A 5% glycerol solution was administered intramuscularly (into the m. gluteus) at a dose of 8–10 ml/kg, twice, with a 24-hour interval between injections. Fourteen days after the experiment began, the animals were sacrificed under anesthesia, and their lung tissues were fixed in a 10% neutral buffered formalin solution for histological examination. The tissues were embedded in paraffin blocks, and sections of 5–7 µm thickness were prepared and stained with hematoxylin and eosin for analysis under a light microscope. Histomorphometric measurements were performed using a digital microscope system, such as the ToupView software.
3. Results and Discussion
- Histological analysis of the lung samples revealed several significant morphological alterations in the CKD group. These included pronounced thickening of the alveolar septa, increased presence of fibroblasts and collagen fibers in the interstitial tissue, collapsed alveoli, and mononuclear inflammatory infiltration. These structural changes represent the pathological basis for impaired pulmonary gas exchange function.The results of this study are consistent with several international sources. For instance, Faulkner et al. (2021) reported thickening of the alveolar walls and interstitial fibrosis in rats with CKD. Another study by Lee et al. (2020) highlighted that uremic toxins and oxidative stress in CKD can lead to apoptosis and inflammation in lung tissue.The use of a 5% glycerol solution in this study enabled the development of a mild yet effective model of chronic kidney disease (CKD), characterized by minimal mortality among the animals and sufficiently distinct histological morphological alterations. This demonstrates the advantages of the experimental model.
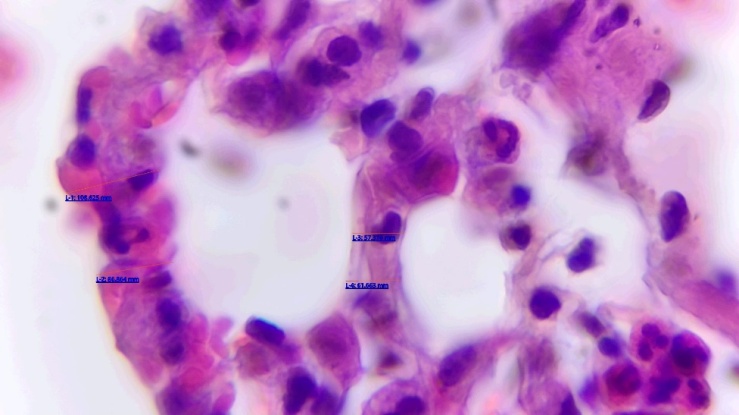 | Figure 1. Histological Image of the Lung of a 5-Month-Old Albino Rat Stained with Hematoxylin and Eosin |
|
|
4. Conclusions
- This experimental study was aimed at identifying the morphological changes occurring in lung tissue under conditions of chronic kidney disease (CKD). The results revealed that thickening of the interalveolar septa, inflammatory infiltration, alveolar deformations, and interstitial fibrosis are key histological features contributing to impaired pulmonary function in the context of CKD. These findings hold significant value for future pathogenesis research and the development of therapeutic strategies.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML
