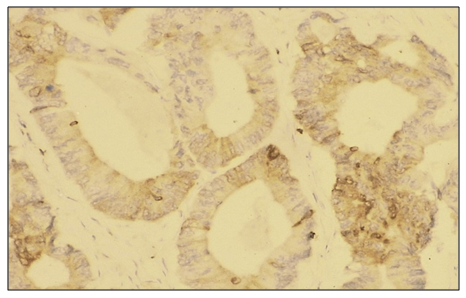
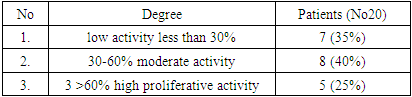
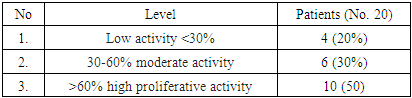
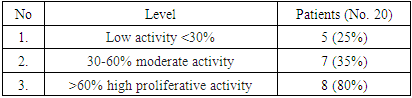
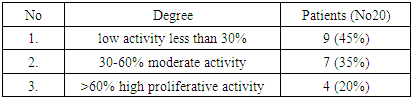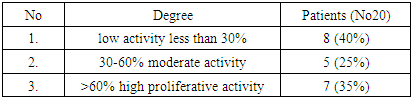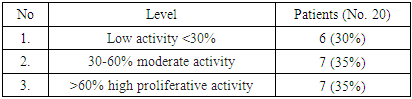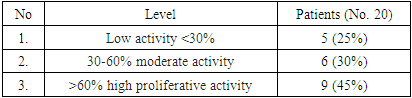| [1] | O.S. Danilova, S.A. Velichko, L.A. Kolomiets, I.G. Frolova, N.G. Truchacheva. "The Role of Radiation Research Methods in the Accurate Diagnosis of Endometrial Cancer in Combination with Metabolic Syndrome," Siberian Oncological Journal, 2012, No. 3 (51). |
| [2] | L.A. Ashrafyan, I.B. Antonova, S.V. Ivashina, N.A. Babayeva, O.A. Alyoshikova, I.I. Baranov. "Optimizing Diagnostic Tactics in Patients with Abnormal Uterine Hemorrhage in Peri- and Postmenopausal Periods," Obstetrics and Gynecology: News, Opinions, Training, 2019, Vol. 7, No. 1, pp. 24-30. doi: 10.24411/2303-9698-2019-11003. |
| [3] | L.F. Gulyayeva, S.E. Krasilnikov. "Molecular mechanisms of endometrial carcinogenesis," Bulletin of the Higher Attestation Commission of the Russian Academy of Medical Sciences, 2012, No. 3 (85), part 1. |
| [4] | N.A. Demakova, O.B. Altukhova, S.P. Pakhomov, V.S. Orlova. "Molecular-genetic mechanisms of endometrial hyperplastic processes development," Medical Series. Pharmacy, 2014, No. 4 (175), issue. 25. |
| [5] | A.B. Muntyan. "Uterine fibroids as a risk factor for endometrial hyperplastic processes and cancer," Siberian Oncological Journal, 2009, appendix. No. 1, p. 140. |
| [6] | A.T. Amiraslanov, S.I. Safarova. "Risk factors and prognostic indicators of atypical endometrial hyperplasia," Bulletin of Modern Clinical Medicine, 2019, vol. 12, issue. 2, pp. 8-9. |
| [7] | R.G. Guseynova, I.M. Ordiants, A.A. Yamurzina, N.L. Sargsyan, S.Ya. Ismailzade. "Malignant Transformation of Hyperplastic Endometrium: Epidemiology and Prognosis," Obstetrics and Gynecology: News, Opinions, Training, 2020, Vol. 8, No. 3, appendix, pp. 100-105. doi: 10.24411/2303-9698-2020-13916. |
| [8] | A.T. Amiraslanov, S.I. Safarova. "Risk factors and prognostic indicators of atypical endometrial hyperplasia," Bulletin of Modern Clinical Medicine, 2019, vol. 12, issue. 2, pp. 8-9. |
| [9] | O.R. Grigoryan, E.N. Andreeva. "Pathogenetic aspects of endometrial hyperplastic processes in women with carbohydrate metabolism disorders in perimenopause," Dr.ru, 2010, No. 7 (58), pp. 39-43. |
| [10] | Yu.S. Sidorenko, E.A. Shurygina, O.G. Shishkina, V.V. Gorobtsova. "Informativeness of some indicators of endocrine homeostasis in the formation of risk groups for the development of endometrial cancer," FSBU RNIOI MZsotsro, Rostov-on-Don, 2012, pp. 83-87. |
| [11] | L.M. Bershtein, A.G. Ievleva, M.S. Mukhina, D.A. Vasiliev, T.E. Poroshina. "Connection of hormone-associated properties and plasticity of omental fat with clinical and morphological features of endometrial cancer," Oncology Questions, 2016, Vol. 62, No. 1, pp. 79-84. |
| [12] | L. Dossus, S. Rinaldi, S. Becker, A. Lukanova, et al. "Obesity, inflammatory markers, and endometrial cancer risk: a prospective case-control study," Endocr Relat Cancer, 2010, vol. 17, no. 4, pp. 1007-1019. |
| [13] | A.L. Chernishova, L.A. Kolomiets, N.V. Yunusova, I.V. Kondakova, A.A. Bulanova, E.V. Shanshashvili. "Pathogenetic justification of the need for correction of metabolic syndrome in patients with endometrial hyperplastic processes and cancer," Russian biotherapeutic journal, 2013, Vol. 12, No. 1, pp. 3-10. |
| [14] | O.N. Asadchikova. "Endometrial cancer in patients with metabolic syndrome: features of the insulin-like growth factor system," Abstract. |
| [15] | A.I. Zhirnyakov. "Metabolic syndrome as a risk factor for the development of endometrial cancer and cardiovascular pathology," Bulletin of Medical Internet Conferences, 2015, Vol. 5, No. 11, pp. 1383-1384. |
| [16] | A.Yu. Kishkina, N.V. Yunusova, L.A. Kolomiets, E.S. Kolegova, I.V. Kondakova. "The Significance of Clinical and Hormonal-Metabolic Parameters in Predicting the Risk of Lymphogenous Metastasis in Patients with Endometrial Cancer in Clinical Stage I," Oncology Questions, 2021, Vol. 67, No. 1, pp. 105-110. |
| [17] | L. Dossus, S. Rinaldi, S. Becker, A. Lukanova, et al. "Obesity, inflammatory markers, and endometrial cancer risk: a prospective case-control study," Endocr Relat Cancer, 2010, vol. 17, no. 4, pp. 1007-1019. |
| [18] | A.Yu. Kishkina, L.A. Kolomiets, N.V. Yunusova. "Clinical variants of metabolic syndrome in patients with endometrial cancer," Siberian Oncological Journal, 2019, Vol. 18, No. 5, pp. 38-44. |
| [19] | K.M. Jurabekova, M.B. Sayfutdinova. "The influence of age-related aspects and metabolic syndrome on the clinical course of endometrial cancer," Payomi Academy of Sciences and Medicine of Tajikistan, 2019, Vol. IX, No. 2, pp. 133-138. |
| [20] | S.B. Radynova, A.G. Kenyaikina, M.A. Turayeva, M.S. Lodyreva. "The Effect of Metabolic Syndrome on Women's Reproductive Function," Medical Sciences / Colloquium-journal, No. 4 (28), 2019, pp. 13-16. |
| [21] | A.Yu. Kishkina, N.V. Yunusova, L.A. Kolomiets, E.S. Kolegova, I.V. Kondakova. "Significance of clinical and hormonal-metabolic parameters in the preoperative prediction of lymphogenous metastasis risk," Oncology Questions, 2021, Vol. 67, No. 1, pp. 105-110. |
| [22] | A.Yu. Kishkina, L.A. Kolomiets, N.V. Yunusova. "Clinical variants of metabolic syndrome in patients with endometrial cancer," Siberian Oncological Journal, 2019, Vol. 18, No. 5, pp. 38-44. |
| [23] | K.M. Jurabekova, M.B. Sayfutdinova. "The influence of age-related aspects and metabolic syndrome on the clinical course of endometrial cancer," Payomi Academy of Sciences and Medicine of Tajikistan, 2019, Vol. IX, No. 2, pp. 133-138. |


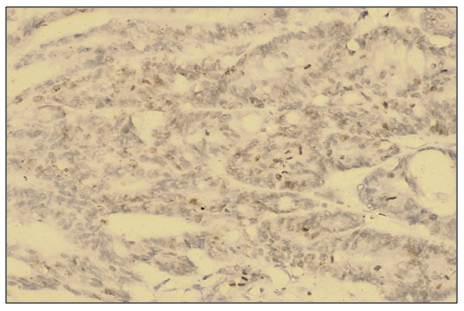
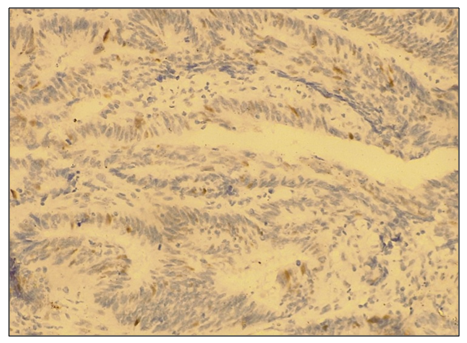
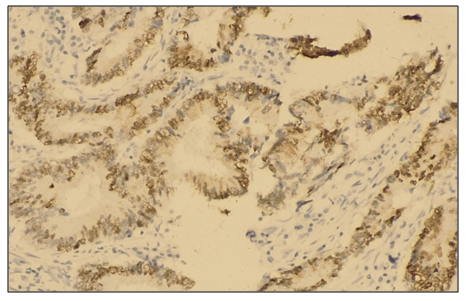
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML