-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(5): 1471-1473
doi:10.5923/j.ajmms.20251505.36
Received: Apr. 19, 2025; Accepted: May 16, 2025; Published: May 24, 2025

Pathomorphology Analysis of the Developing Changes in Myometrial Structures After Pregnancy in Women with Uterine Fibromatosis
Jumanov Ziyadulla Eshmamatovich1, Sharipov Oybek Turgunovich2
1Professor, Doctor of Medical Sciences, Department of Pathological Anatomy, Samarkand State Medical University, Uzbekistan
2Independent Researcher of the Department of Pathological Anatomy of Samarkand State Medical University, Uzbekistan
Correspondence to: Jumanov Ziyadulla Eshmamatovich, Professor, Doctor of Medical Sciences, Department of Pathological Anatomy, Samarkand State Medical University, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In order to conduct a pathomorphological analysis of the changes that develop in the myometrial structures after pregnancy in women with uterine fibromatosis, tissue fragments taken from the uterus, which were extrapolated due to uterine fibromatosis, were subjected to pathomorphological examination in 42 pregnant women. It was found that uterine fibromatosis can occur in women of different ages and with different numbers of pregnancies. Dystrophic, necrotic and sclerotic processes are noted in the myometrial tissues located close to the areas of fibromatosis. Lymphocytic infiltration is also observed in the areas of connective tissue growth.
Keywords: Uterus, Pregnancy, Dystrophy, Necrosis, Edema, Fibromatosis, Analysis
Cite this paper: Jumanov Ziyadulla Eshmamatovich, Sharipov Oybek Turgunovich, Pathomorphology Analysis of the Developing Changes in Myometrial Structures After Pregnancy in Women with Uterine Fibromatosis, American Journal of Medicine and Medical Sciences, Vol. 15 No. 5, 2025, pp. 1471-1473. doi: 10.5923/j.ajmms.20251505.36.
1. Introduction
- Uterine fibromatosis is a very common gynecological disease in women of reproductive age. This is a pathological process in which the division of muscle layer cells is accelerated and they are replaced by altered connective tissue, which differs from healthy tissues in a denser structure and reduced contractility [1]. Fibromatous nodes are benign neoplasms, the percentage of their transformation into malignant tumors is almost zero. A complication of the pathology is the formation of large fibroids and fibroids, which cause uterine bleeding, pain and discomfort in the abdomen and lower back, and problems during pregnancy. They prevent the attachment of a fertilized egg, leading to miscarriage [4,6]. Uterine fibromatosis can regress during menopause, since during this period the female sex hormones cease to be produced in large quantities. The development of fibromatosis during menopause is associated with the use of hormonal drugs to improve the patient's condition. Fibroids and myoma complications during menopause can be treated with uterine artery embolization [2,3,5]. Uterine fibroids or leiomyomas are the most common benign tumors of the uterus in women of childbearing age, and their etiology is not fully understood to date. These tumors grow rapidly and cause a certain degree of myometrial tissue alteration. They also lead to the development of pathophysiological conditions. Ultrasound examination has shown that the prevalence of uterine fibroids has increased from approximately 35% (clinical diagnosis only) to approximately 50% of fertile women (ultrasound diagnosis) [7]. The incidence of uterine fibroids increases to approximately 80% after hysterectomy [8].The purpose of the study: To conduct a pathomorphological analysis of changes that develop in myometrial structures after pregnancy in women with uterine fibroids.
2. Materials and Methods
- The uterus, which was extrapolated due to uterine corpus fibromatosis in 42 pregnant women, was studied at the Department of Pathological Anatomy of the Multidisciplinary Clinic of the Samarakand State Medical University. Pathomorphological assessment of changes in the structures of the uterine myometrium layer was carried out using anamnestic, macroscopic, microscopic, morphometric and statistical research methods. Histological sections were stained with hematoxylin-eosin. Microphotographic techniques were conducted.Histological preparations were studied and photographed using a LeicaGME microscope (Leica, India) coupled with a LeicaEC3 digital camera (Leica, Singapore) and a Pentium IV computer. Photo processing was carried out using Windows Professional applications.
3. Results and Discussion
- The results of the study show that 35 women who were removed due to uterine fibroids were of different ages, 10 of whom were aged 35-40 years old, with an average number of pregnancies and deliveries of 2-3. 21 were aged 40-45 years old, with an average number of pregnancies of 3-5, with an average number of deliveries of 3. The remaining 3 were women over 45 years old, with an average number of pregnancies of 5-6, and with an average number of deliveries of 3-4. Histological examination of micropreparations prepared from uterine myometrial tissue revealed specific changes in the structures of the myometrial layer located around the fibroids. In particular, intermuscular edema, hydropic dystrophy in myocytes, and karyopyknotic processes in the nucleus were detected. In particular, the presence of collagen fibers and a small number of fibroblasts and lymphocytes between myocytes is detected (Figure 1).
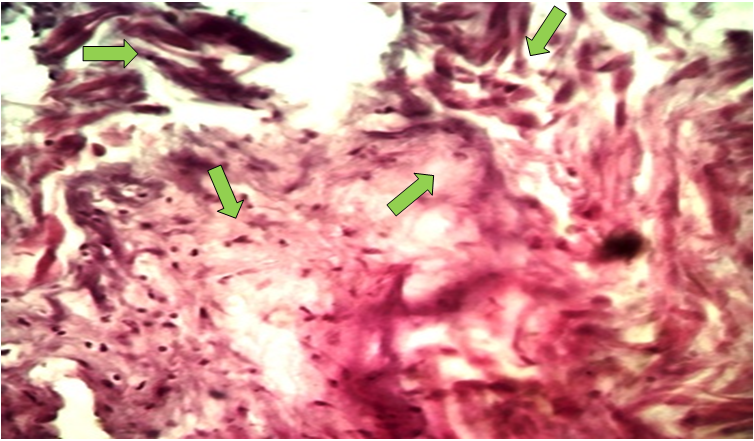 | Figure 1. Uterine fibromatosis. Intermuscular edema. Fibrocyte proliferation. Fibrous tissue. Hematoxylin-eosin stained. Ob.40, ok.10 |
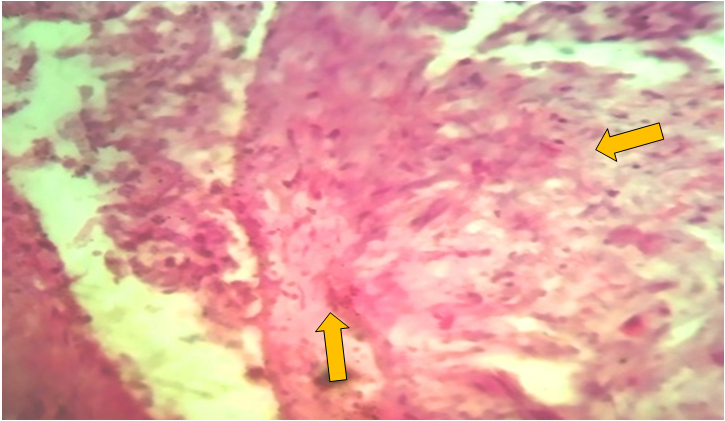 | Figure 2. Fibromatous changes in the blood vessels of the uterine body. Stained with hematoxylin-eosin. Ob.40, ok.10 |
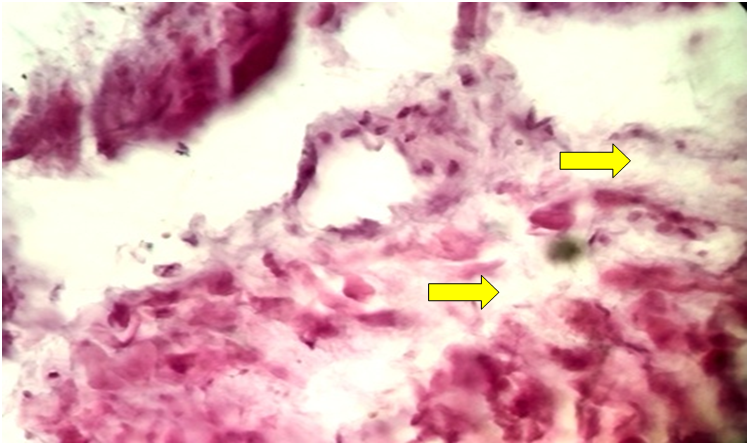 | Figure 3. Fibromatous changes in the blood vessels of the uterine myometrium replacing the muscles. Stained with hematoxylin-eosin. Ob.40, ok.10 |
4. Conclusions
- Thus, uterine fibroids can occur in women of different ages and with different numbers of pregnancies and deliveries. Dystrophic, necrotic and sclerotic processes are noted in the myometrial tissues located near the areas of fibromatous changes. Lymphocytic infiltration is also observed in areas of connective tissue growth. In most blood vessels near the areas of fibromatosis, spasms and narrowing of the lumen due to sclerotic processes in the walls are noted. In addition, it is determined that the fibromatous process that has developed in the walls of many blood vessels has replaced the muscles of the myometrial layer. This condition should be taken into account in the pre-pregnancy examinations of women of childbearing age.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML