-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(5): 1448-1450
doi:10.5923/j.ajmms.20251505.28
Received: Apr. 20, 2025; Accepted: May 16, 2025; Published: May 24, 2025

Specific Aspects of Morphological Changes in the Adrenal Glands of Infants Who Died Due to Toxoplasmosis Infection
Djumanova Gulchekhra Eshmamatovna1, Mamadiyarova Dilfuza Umirzakovna2
1Independent Researcher of the Department of Pathological Anatomy of Samarkand State Medical University, Uzbekistan
2Associate Professor, Department of Pedagogy and Psychology, Samarkand State Medical University, Uzbekistan
Correspondence to: Djumanova Gulchekhra Eshmamatovna, Independent Researcher of the Department of Pathological Anatomy of Samarkand State Medical University, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Anamnestic, macroscopic, microscopic examination of 80 infants who died of toxoplasmosis infection, morphological analysis of micropreparations stained with the hemotoxylin-eosin method from sections of the adrenal glands were conducted. It was found that infants with toxoplasmosis infection develop necrotic and necrobiotic changes in the adrenal gland. It is emphasized that this should be taken into account when detecting toxoplasmosis infection in pregnant women.
Keywords: Adrenal gland, Toxoplasmosis, Endocrinocyte, Karyopycnosis, Karyorexic
Cite this paper: Djumanova Gulchekhra Eshmamatovna, Mamadiyarova Dilfuza Umirzakovna, Specific Aspects of Morphological Changes in the Adrenal Glands of Infants Who Died Due to Toxoplasmosis Infection, American Journal of Medicine and Medical Sciences, Vol. 15 No. 5, 2025, pp. 1448-1450. doi: 10.5923/j.ajmms.20251505.28.
1. Introduction
- More than half of the world's population is infected with toxoplasmosis, with 23% of the USA population carrying the parasite, about 20% in Russia, and as many as 95% in some countries. The annual incidence of toxoplasmosis is estimated at 190,100 cases worldwide [4,11,12,16]. If a pregnant woman has toxoplasmosis, it is transmitted to the fetus through the placenta in 30% of cases. Congenital toxoplasmosis is the second most common intrauterine infection after cytomegalovirus infection [7,8,10]. Toxoplasmosis is a severe protozoan disease of humans and animals caused by an obligate intracellular parasite with a complex development cycle, Toxoplasma gondii, which has a predominantly chronic latent course and occurs with signs of damage to the nervous system, organs of the reticuloendothelial system, striated muscles and the organ of vision [1,2,3]. In 1972, experts from the World Health Organization included toxoplasmosis in the list of zoonoses that are most dangerous to human health, and in the late 1980s it was recognized as one of the few opportunistic infections of protozoan etiology. Toxoplasmosis is a ubiquitous (ubiquitous) disease that occurs on all continents, in countries with different climatogeographic conditions. This distinguishes it from other zoonotic diseases that have a strictly defined area. The ability of toxoplasmas to infect a huge number (more than 300) of domestic and wild animal species living in different landscape zones causes the infection to spread widely among the population of all countries [5,6,9,15]. The extremely wide prevalence of the invasion and the heterogeneity of the human population in terms of the state of the immune system cause a significant number of patients with manifest forms of toxoplasmosis. In the United States, the total number of cases of Toxoplasma gondii infection is 1.5 million people annually, with 150,000 to 300,000 cases being clinically significant [13,16].The purpose of the study: Identification of pathomorphological changes in the adrenal gland under the influence of toxoplasmosis infection.
2. Materials and Methods
- Anamnestic, macroscopic, microscopic examination of 80 infants who died due to toxoplasmosis infection was performed in the Department of Pathological Anatomy of the Multidisciplinary Clinic of the Samarkand State Medical University. In the study, tissue fragments of 1x1x0.5 cm were taken from the wall of the left ventricle of the heart of the babies who were born prematurely and lived up to 3 days. The obtained tissue fragments were fixed in 10% neutral formalin, passed through an alcohol battery, and paraffin blocks were prepared. The prepared histological sections were stained with hematoxylin and eosin, according to Van-Gieson. Microphotographic techniques were conducted. The number of infants who died from the intrauterine form of toxoplasmosis infection was 81, of which 64 were boys (79%) and 17 were girls (21%). These infants are stillborn in the last trimester of pregnancy. Histological preparations were studied and photographed using a LeicaGME microscope (Leica, India) coupled with a LeicaEC3 digital camera (Leica, Singapore) and a Pentium IV computer. Photo processing was carried out using Windows Professional applications.
3. Results and Discussion
- The results of the analysis show that the connective tissue membrane of the adrenal gland of stillborn babies is thickened, the connective tissue is swollen, the adipocytes located in its spaces are clustered and their size is reduced due to non-folding (61±0.4 μm), the blood vessels are full, diapedetic hemorrhages are detected. The cytoplasm of the cubic adrenocorticocytes located in the glomeruli of the cortical layer of the adrenal gland is swollen, karyopycnosis and karyorrhexis are observed in the nucleus, and karyolysis is observed in some cells (Figure 1). Therefore, the thickness of this layer is on average 21±2.2 μm. In the entire section, swelling is detected in cubic and prismatic endocrinocytes and karyopycnosis and karyorrhexis in the nucleus.
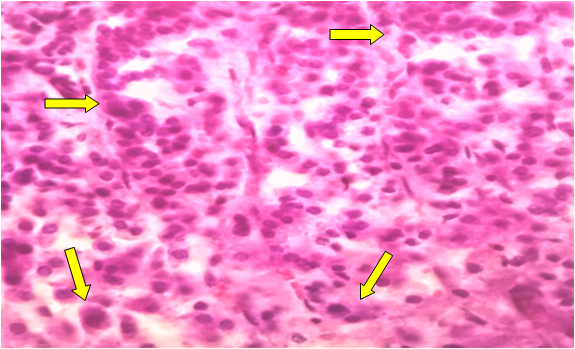 | Figure 1. Dystrophic changes in cuboidal adrenocorticocytes located in the glomeruli of the adrenal cortex. Stained with hematoxylin-eosin. Ob.40, ok.10 |
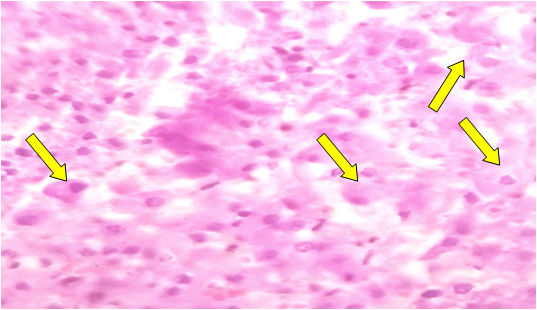 | Figure 2. Dystrophic changes in adrenolinocytes and noradrenolinocytes of the medullary layer of the adrenal gland. Stained with hematoxylin-eosin. Ob.40, ok.10 |
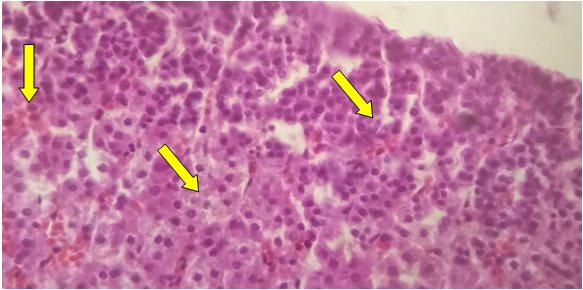 | Figure 3. Dystrophic changes in the medullary cells of the adrenal gland and diapedetic hemorrhages. Stained with hematoxylin-eosin. Ob.40, ok.10 |
4. Conclusions
- In infants with toxoplasmosis infection, the cytoplasm of cubic adrenocorticocytes located in the glomeruli of the adrenal cortex is swollen, karyopycnosis and karyorrhexis are observed in the nucleus, and karyolysis is observed in some cells. In the reticular part, swelling is detected in cubic and prismatic endocrinocytes, and karyopycnosis and karyorrhexis in the nucleus. Endocrinocytes in the reticular part are sparsely located, swelling is detected in the cytoplasm, karyopycnosis and karyorrhexis in the nucleus, and karyolysis is observed in some cells. In the medullary layer, adrenolinocytes and noradrenolinocytes are round in shape, larger in size, and sparsely located. This should be taken into account when diagnosing toxoplasmosis infection in pregnant women.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML