-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(5): 1411-1413
doi:10.5923/j.ajmms.20251505.20
Received: Apr. 26, 2025; Accepted: May 12, 2025; Published: May 15, 2025

Immunological Aspects of Renal Dysfunction in Post-Covid Syndrome
Khaytboev Jurabek Azatboyevich, Rakhmanova Sanobar Sobirovna
Urgench Branch of Tashkent Medical Academy, Khorezm, Uzbekistan
Correspondence to: Khaytboev Jurabek Azatboyevich, Urgench Branch of Tashkent Medical Academy, Khorezm, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Post-covid syndrome directly and indirectly affects renal function, leading to the development of acute and chronic renal complications. Factors such as inflammatory response, endothelial dysfunction, hypoxia and hypercoagulability, including the “cytokine storm” specific to Covid-19, play an important role in the pathogenesis of these complications.
Keywords: Post-covid syndrome, Renal complications, Immunology
Cite this paper: Khaytboev Jurabek Azatboyevich, Rakhmanova Sanobar Sobirovna, Immunological Aspects of Renal Dysfunction in Post-Covid Syndrome, American Journal of Medicine and Medical Sciences, Vol. 15 No. 5, 2025, pp. 1411-1413. doi: 10.5923/j.ajmms.20251505.20.
Article Outline
1. Introduction
- Numerous studies conducted in different countries of the world have shown that cases of renal dysfunction were observed in the post-Covid-19 period among patients infected with SARS-CoV-2, and the characteristic features of these diseases were a decrease in glomerular filtration rate parameters [1]. However, to date, it has not been proven that the cause of renal dysfunction after Covid-19 is the virus, drug toxicity, or molecular genetic aspects [2]. It is important to determine how the respiratory virus directly disrupts renal function, as well as to assess the impact of immunological aspects and their role and significance in the mechanism of the emergence of post-Covid-19 renal dysfunction [3]. The post-infection consequences of Covid-19 or post-Covid syndrome (PCS) affect several internal organ systems, including the kidneys. This poses the medical community with the issue of solving problems related to the treatment and diagnostic tactics of renal complications [4].Thus, a lot of scientific research is currently being conducted around the world to ensure timely and accurate diagnosis and treatment of coronavirus, as well as to identify the unpleasant condition of post-covid syndrome [5].Although the novel coronavirus Covid-19, caused by the SARS-CoV-2 virus, primarily affects the lungs, in some patients the infection also spreads to other organ systems [6]. Recently, there has been a significant increase in cases of kidney dysfunction associated with previous Covid-19 infection [7]. Studies to date show that Covid-19 has a wide range of symptoms and the long-term consequences for people with severe and very severe disease can be life-threatening. Such conditions include pulmonary sclerosis and kidney failure, inflammation of the heart muscle, arrhythmia, liver damage, cognitive impairment, psychosis accompanied by sharp mood swings, etc. [8].Like other respiratory viruses such as MERS-CoV, the scientific medical community paid little attention to the consequences of Covid-19 and viewed it as a common respiratory disease. However, by 2021, a year after the start of the pandemic, it became clear that the SARS-CoV-2 virus affects the lungs, brain, nasopharynx, eyes, heart, blood vessels, liver, kidneys and intestines, literally affecting all vital organs.Numerous studies conducted in different countries of the world have shown that there were cases of renal dysfunction in the post-Covid-19 period among patients infected with SARS-CoV-2, and the characteristic features of these diseases were a decrease in the level of glomerular filtration rate parameters. However, to date, it has not been proven that the cause of renal dysfunction after Covid-19 is viral tropism, drug toxicity, or molecular genetic aspects. It is important to determine how the respiratory virus directly disrupts kidney function, as well as to assess the impact of immunological aspects and their role and significance in the mechanism of occurrence of renal dysfunction after Covid-19.
2. Purpose of the Research
- The purpose of the study is to identify and diagnose the most important prognostic factors of this pathology based on a diagnostic study of immunological status parameters in patients with impaired renal function after Covid-19.
3. Materials and Methods
- In the observational diagnostic study, 240 patients who had experienced Covid-19 and had symptoms of renal failure were selected. They were divided into 2 groups according to several disease symptoms for 6 months, and the analysis of clinical and laboratory data was studied: The main group included 120 patients with signs of a decrease in glomerular filtration rate (GFR) <90.0 ml/min/1.73 m2 within 6 months after Covid-19; The control group included 120 patients with normal GFR levels during or after Covid-19 for 6 months. Of these, 116 (48.3%) were women and 124 (51.7%) were men aged 23 to 76 years, with a median age of 44.5 years in the interquartile range (IQR-interquartile range): 35-56 years. Body mass index (BMI) ranged from 23.4 to 34.3 kg/m2, median 28.3 kg/m2, IQR: 26.4-30.2 kg/m2. The study participants included 25 (10.4%) underweight, 121 (50.4%) normal weight, 64 (26.7%) overweight, and 30 (12.5%) obese patients. Of the study participants, 202 (84.2%) had only 1 episode of Covid-19, and 38 (15.8%) had more than 1 episode of Covid-19 before developing post-covid syndrome (PCS).From the laboratory markers of the immunological profile, we analyzed Interleukin-2 (IL-2), Interleukin-5 (IL-5) using an enzyme-linked immunosorbent assay (ELISA) of a chromogenic substrate that binds antigens and antibodies to these cytokines, as well as the cytokines Interleukin-6 (IL-6), Interleukin-8 (IL-8) Interleukin-10 (IL-10) and Interleukin-18 (IL-18) in serum in the first stages of the study, and IL-6 was again determined in order to assess systemic damage and inflammation. These interleukins are key mediators of the processes associated with inflammation and the cytokine storm in Covid-19. Thus, IL-2 is involved in the activation and proliferation of T-lymphocytes, which are important for maintaining cellular immunity; IL-5 regulates eosinophil function, which may be associated with inflammatory changes in the renal vascular system; IL-6 is one of the main pro-inflammatory mediators, the levels of which are significantly increased in severe Covid-19, associated with the development of a cytokine storm; IL-8 promotes the accumulation of neutrophils at the site of inflammation, which can lead to tissue damage and organ dysfunction. Interleukin-10 (IL-10), an anti-inflammatory cytokine, suppresses immune function by blocking the synthesis of proinflammatory cytokines (e.g., IL-1, IL-6, IFN-γ, and TNF-α) in T cells, monocytes, and macrophages, and by inhibiting the expression of cell surface molecules involved in antigen presentation and stimulation. These biological functions suggest IL-10 may have utility in the treatment of autoimmune and inflammatory diseases. Interleukin-18 (IL-18) is a potent pro-inflammatory cytokine involved in host defense against infections and regulates the innate and acquired immune response. IL-18 is produced by both hematopoietic and non-hematopoietic cells, including monocytes, macrophages, keratinocytes and mesenchymal cell. IL-18 could potentially induce inflammatory and cytotoxic immune cell activities leading to autoimmunity.
4. Results and Discussion
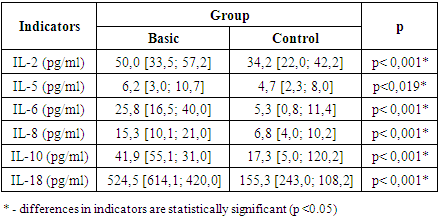
- The most pronounced changes were observed in the level of IL-6, the average of which in patients in the main group was 25.8 pg/ml, which is 79.4% higher than the values in the control group (5.3 pg/ml, p<0.001). These data confirm the key role of IL-6 in systemic inflammation and kidney tissue damage in the post-Covid period. Significant differences were also found in the level of IL-2, IL-5 and IL-8. In patients with renal failure, the average level of IL-2 was 50.0 pg/ml, which is 31.6% higher than in the control group (34.2 pg/ml, p<0.001), the level of IL-5 increased by 24.1% (4.7 pg/ml versus 6.2 pg/ml, p=0.019). The increase in IL-8 levels reached 55.5% (15.3 pg/ml versus 6.8 pg/ml, p<0.001), highlighting its role in maintaining the inflammatory process (Table 1).
|
5. Conclusions
- The prevalence of nephrological complications in patients with Covid-19 in the Khorezm region was 22.7%. For every 5 patients with post-covid syndrome, 2 had impaired renal function (rxy=0.943; p=0.057). In patients with post-COVID renal dysfunction, a significant decrease in GFR to 69.2 ml/min/1.73 m² (p<0.001) (in the control group it was 96.4 ml/min/1.73 m²), creatinine (mean 125 μmol/l vs. 71 μmol/l, p<0.001), S-reactive protein (3.8 mg/dl vs. 2.3 mg/dl, p<0.001) and ferritin (304.8 mg/dl vs. 201.8 mg/dl, p<0.001), proteinuria (149.6 mg/dl vs. 99.8 mg/dl, p<0.001) and albuminuria (30.7 mg/l vs. 5.5 mg/l, p<0.001) were observed; The risk of developing post-COVID renal dysfunction was increased by 4.448 times (p<0.001) by repeated episodes of Covid-19 disease, 3.575 times (p<0.001) by the absence of vaccination against SARS-CoV-2, and 2.532 times (p<0.001) by severe course, while the presence of harmful habits increased the likelihood of nephrological complications by 2.275 times (p<0.001). Gender, age, and body mass index and the use of SOV did not have a statistically significant effect (p=0.996; p=1.000; p=0.951; p=0.982);In the main group, compared to the control group, the level of IL-18 was 3.377 times higher (mean 524.5 pg/ml and 155.3 pg/ml, p<0.001), the level of IL-10 was 2.421 times higher (mean 41.9 pg/ml and 17.3 pg/ml, p<0.001), the level of IL-6 was 2.504 times higher (mean 25.8 pg/ml and 10.3 pg/ml, p<0.001), the level of IL-8 was 2.250 times higher (15.3 pg/ml and 6.8 pg/ml, p<0.001), the level of IL-2 was 1.389 times higher (18.2 pg/ml and 13.1 pg/ml, p<0.001), the level of IL-5 was 1.319 times higher (6.2 pg/ml and 4.7 pg/ml, p<0.001). p=0.019). This confirms the hypothesis that the mechanism of renal dysfunction after Covid-19 depends on characteristic cytokines (p<0.05).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML