-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(5): 1394-1397
doi:10.5923/j.ajmms.20251505.16
Received: Apr. 22, 2025; Accepted: May 11, 2025; Published: May 13, 2025

Morphological Changes in the Small Intestine Wall in Experimental 3-Month-Old White Breed Rats with Burns of the Digestive Tract Caused by Acetic Acid
Khamroeva Lola Rizoevna
Department of Anatomy and Clinical Anatomy (OXTA), Bukhara State Medical Institute, Uzbekistan
Correspondence to: Khamroeva Lola Rizoevna, Department of Anatomy and Clinical Anatomy (OXTA), Bukhara State Medical Institute, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In case of chemical burns, gastrointestinal and respiratory burns can be caused by accidental or suicidal use of the substance. The circumstances of the accident and the chemical nature of the substance determine the degree of damage and the toxicological risk. The early period after a chemical burn is characterized by swelling of the larynx, perforation of the esophagus, stomach and intestines, gastrointestinal bleeding and the possibility of pancreatitis.
Keywords: Chemical burn, Gastrointestinal tract, Resorption, Moxibustion
Cite this paper: Khamroeva Lola Rizoevna, Morphological Changes in the Small Intestine Wall in Experimental 3-Month-Old White Breed Rats with Burns of the Digestive Tract Caused by Acetic Acid, American Journal of Medicine and Medical Sciences, Vol. 15 No. 5, 2025, pp. 1394-1397. doi: 10.5923/j.ajmms.20251505.16.
Article Outline
1. Introduction
- Active ongoing research in the study of chemical burns of the gastrointestinal tract confirms the complexity of this problem and its relevance. According to the American Association of Poison Control Centers, more than 1.6 million child poisoning occurred in 2008 alone, with alkaline esophageal burns recorded in 18-46% of cases after consuming various household chemicals. In chemical burns of the digestive tube with acetic acid, accidental or suicide of the substance can cause gastrointestinal and respiratory burns. The circumstances of the accident and the chemical nature of the substance determine the degree of damage and toxicological risk. The initial period after chemical burns is associated with the possibility of laryngeal edema, perforation of the esophagus, stomach and intestines, gastrointestinal bleeding, and pancreatitis.Currently, chemical burns of various degrees of digestion are a very urgent medical, social and economic problem. Severe burns of the upper gastrointestinal tract affect 10-33% of adult patients, the mortality rate is observed up to 10%.Two age groups are at the greatest risk: children aged 2-6 years, who involuntarily consume household cleaning products and burn up to 80% of ingestion, but usually with mild injuries; and adults aged 30-40 years who use strong corrosive substances with the intention of suicide and who resort to serious, life-threatening injuries. Suicidal attempts predominate in women as reasons for taking burning fluids. Most often they are observed in young women and are seasonal (spring and autumn). In some patients, knowing the possible death and the nature of the fluid causes fear. Patients who decide to commit serious suicide take a large amount, more than 50-100 ml of burning fluid in one sip, and they experience deep burns not only of the oral cavity, throat and esophagus, but also of the stomach, duodenum and hungry intestine. Such patients die of severe general intoxication and extensive tissue necrosis within the first two to three days. Severe burns can not only cause extensive damage to the skin and subcutaneous soft tissues, but also cause damage to many organs due to ischemia, hypoxia and inflammation, and the intestine is one of the most vulnerable organs to burn damage. Burn injury significantly reduces blood perfusion in the intestine, as a result of which the structure and function of the intestinal mucosa is disrupted. The intestinal mucosa is the main place responsible for the digestion and absorption of food, and its barrier function is also the main element responsible for maintaining the ecological stability of the body.Damage to the intestinal mucosa barrier after a burn injury can lead to the migration of bacteria and toxins, and this translocation can lead to intestinal infections and upper intestinal metabolism, which leads to a poor prognosis. Studies have shown that loss of the mucous barrier can exacerbate intestinal damage caused by various pathological factors. Failure of mucous secretion under various pathological conditions and damage to the intestinal mucosa as a result of changes in mucous composition are some of the starting factors leading to intestinal damage. The late consequences of burns include retrograde changes in the oral cavity, esophagus, stomach or respiratory system. In addition, this can lead to the formation of strictures in the organs. The formation of stricture can have serious systemic consequences for the patient, including poor overall condition, significant weight loss, malnutrition-related diseases, repeated aspiration leading to respiratory infections, and potential respiratory failure. To assess the severity of the injury in patients with acetic acid poisoning, it is recommended to determine the presence and level of free hemoglobin in the blood and urine. [1]
2. The Purpose of the Study
- White breed rats digest what to use the biological method of correction using black sedana oil in order to detect trophological changes in the wall of the small intestine in burns caused by various degrees of oxalic acid, to reduce the side effects of the chemical substance.
3. Research Materials and Methods
- For experimental purposes, 120 white non-breeding bats of both sexes were used, which were kept in normal vivarium conditions of 3 months. All experimental rats were kept in vivarium in plastic cages, at room temperature, in accordance with the standards for keeping laboratory animals. At the beginning of the experiment, all rats were quarantined for a week, and after the elimination of somatic or infectious diseases, they were transferred to a normal vivarium regime. Based on the model developed in the study on the chemical burn of white-breed rats digested nai No. 2269349, a61k 35/00, a61n 5/067, a61r 1/00, publ. 10.02.2006 held. In the experiment, the animals of the experimental group were knocked unconscious under general narcosis using a general ether, a solution of acetic acid (70% of the total dose) was sent through an 8 cm probe with a diameter of 1.5 ml. Animals were divided into 3 groups (n=110): Group I-3 months of Control (n=25); Group II rats with a 3 – month (total dose of 70% li) dose of acetic acid (n=40); Group III - 3 months (total dose 70%,) after receiving a dose of acetic acid, rats were ingested through a black sedana oil probe for 30 days (n=45); [2].
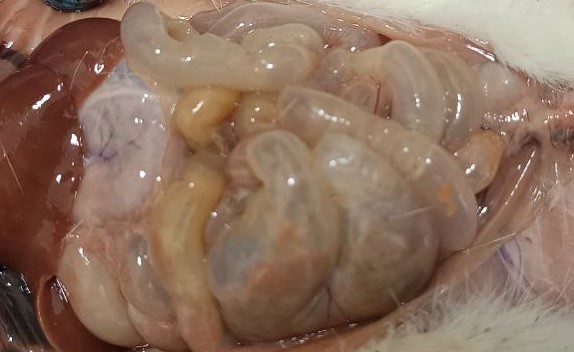 | Figure 1. 70% acetic acid of the small intestine of a 3-way white-breed rat macroscopic appearance after exposure |
4. Conclusions
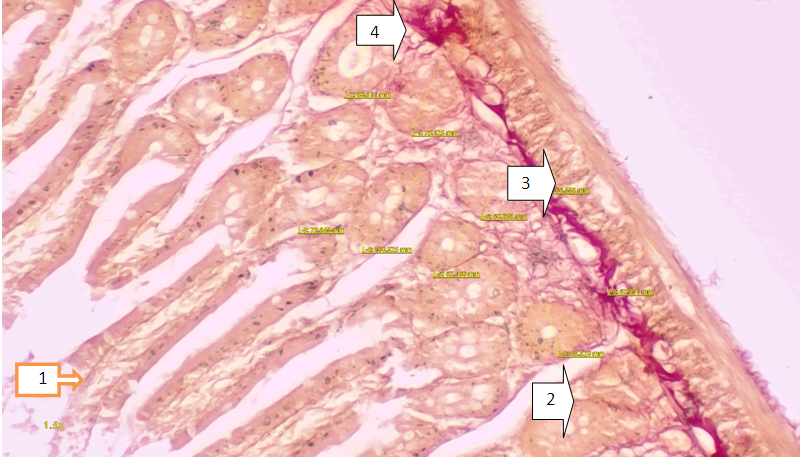
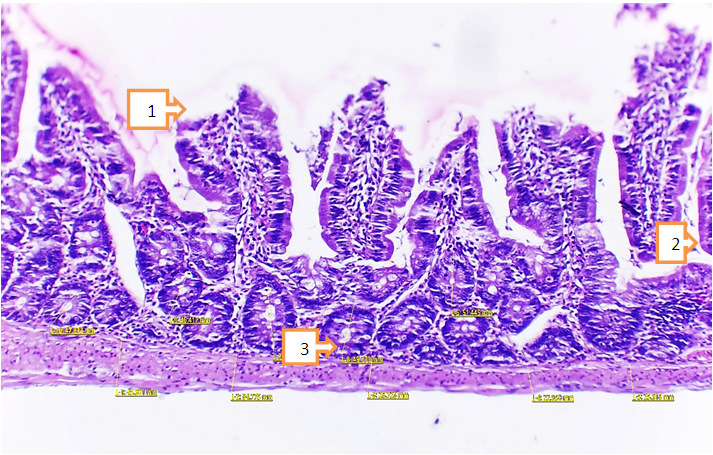
- Conclusion the following morphological changes in the small intestine in chemical burns of the experimental gastrointestinal tract were found, including furnace atrophy of the intestinal vortices, dystrophic and necrobiotic changes of the crypts and versine, reduced musculature, the appearance of signs of inflammation in the intestinal crypts, signs of inflammation in the inner circular floor as well as swelling, signs of infiltration Their detection indicates dystrophic changes in the small intestine, an inflammatory process.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML