-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(5): 1354-1358
doi:10.5923/j.ajmms.20251505.08
Received: Apr. 7, 2025; Accepted: Apr. 23, 2025; Published: May 8, 2025

Morphology and Morphometric Indices of the Ovary and Uterus in White Laboratory Rats During Postnatal Ontogenesis
Dilorom Adilbekovа 1, Sevara Kuranbaeva 2, Malokhat Nazarova 3
1Professor, Department of Anatomy and Clinical Anatomy, Tashkent Medical Academy, Tashkent, Uzbekistan
2Assistant, Department of Human Anatomy and Clinical Anatomy, Urgench branch of the Tashkent Medical Academy, Khorezm, Uzbekistan
3Assistant, Department of Pediatrics and Higher Nursing, Urgench branch of Tashkent Medical Academy, Khorezm, Uzbekistan
Correspondence to: Dilorom Adilbekovа , Professor, Department of Anatomy and Clinical Anatomy, Tashkent Medical Academy, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The study of ovarian and uterine morphology and morphometric parameters during postnatal ontogenesis is essential for understanding reproductive development in mammals. This research investigates the structural and dimensional changes in the ovaries and uterus of white laboratory rats (Rattus norvegicus) from birth to sexual maturity. Histological and morphometric analyses were conducted at key developmental stages (neonatal, prepubertal, pubertal, and adult phases). The results demonstrate progressive growth in ovarian follicle populations, with primordial follicles dominating early stages and Graafian follicles appearing at puberty. Uterine morphometry revealed significant increases in wall thickness, luminal diameter, and glandular development post-puberty. These findings contribute to comparative reproductive biology and provide a foundation for further studies on mammalian reproductive organ development.
Keywords: Postnatal ontogenesis, Reproductive biology, White laboratory rats (Rattus norvegicus), Morphology, Morphometry, Histology
Cite this paper: Dilorom Adilbekovа , Sevara Kuranbaeva , Malokhat Nazarova , Morphology and Morphometric Indices of the Ovary and Uterus in White Laboratory Rats During Postnatal Ontogenesis, American Journal of Medicine and Medical Sciences, Vol. 15 No. 5, 2025, pp. 1354-1358. doi: 10.5923/j.ajmms.20251505.08.
Article Outline
1. Introduction
- The study of ovarian and uterine morphology and morphometric parameters during postnatal ontogenesis is crucial for understanding the fundamental processes of reproductive development in mammals. White laboratory rats (Rattus norvegicus) have long served as a primary model in reproductive biology due to their physiological similarities to humans, short reproductive cycles, and well-documented developmental stages. The postnatal development of the ovaries and uterus involves complex structural and functional transformations that are tightly regulated by hormonal, genetic, and environmental factors. Researchers were among the first to systematically describe follicular dynamics in rodent ovaries, demonstrating that folliculogenesis begins prenatally but undergoes significant maturation after birth [1]. Subsequent studies further elucidated the mechanisms of follicular recruitment and atresia, highlighting the importance of gonadotropins in regulating ovarian development [2].The uterus, similarly, undergoes dramatic changes from birth to sexual maturity. Classic works outlined the stages of uterine differentiation, emphasizing the role of estrogen in endometrial proliferation and gland formation [3]. More recent investigations have expanded our understanding of how early-life exposures can alter uterine morphometry, with implications for fertility and reproductive disorders [4]. Additionally, studies provided foundational knowledge on the hormonal regulation of uterine growth during postnatal development [5,6].Despite these advances, comprehensive morphometric analyses of both the ovary and uterus across key developmental stages—neonatal, prepubertal, juvenile, pubertal, and adult—remain limited. Most existing studies focus either on isolated time points or only one reproductive organ, leaving gaps in our understanding of their coordinated development. For instance, while the work of detailed follicular counts in immature rats, it did not correlate these findings with concurrent uterine changes [7]. Similarly, research provided detailed uterine morphometrics but lacked comparative ovarian data [8].This study aims to bridge these gaps by conducting a systematic evaluation of both ovarian and uterine morphology and morphometric indices in white laboratory rats throughout postnatal ontogenesis. By integrating histological and quantitative analyses, we seek to establish a reference framework for normal developmental patterns, which can serve as a baseline for future studies on reproductive toxicology, endocrine disruption, and comparative mammalian biology. Our findings will contribute to the broader understanding of reproductive organ maturation, building upon the foundational work of earlier researchers while providing new insights into the dynamic interplay between ovarian folliculogenesis and uterine structural differentiation.
2. Purpose of the Research
- The purpose of this research is to conduct a comprehensive morphological and morphometric analysis of the ovaries and uterus in white laboratory rats throughout postnatal ontogenesis, from the neonatal period to sexual maturity. By systematically examining structural changes and quantitative parameters at key developmental stages (newborn, prepubertal, juvenile, pubertal, and adult), this study aims to establish normative reference data for reproductive organ development in this widely used experimental model. The research seeks to characterize the progression of folliculogenesis in the ovaries, including the dynamics of primordial, primary, secondary, antral, and Graafian follicle populations, while simultaneously documenting the corresponding histological and dimensional changes in uterine tissues, such as endometrial thickness, myometrial development, luminal diameter, and glandular differentiation. Additionally, this investigation intends to correlate ovarian and uterine developmental patterns to better understand the temporal relationship between follicular maturation and uterine preparation for potential pregnancy. The findings will provide valuable baseline data for comparative reproductive biology studies and serve as a foundation for future research in reproductive toxicology, endocrine disruption, and developmental biology, while also contributing to the validation of the rat model for studies of mammalian reproductive system development. Furthermore, the study aims to identify critical windows of reproductive organ development that may be particularly vulnerable to environmental or pharmacological influences, thereby enhancing our understanding of factors that could impact fertility and reproductive health.
3. Materials and Methods
- The study utilized 60 female white laboratory rats (Rattus norvegicus) divided into five age groups representing key developmental stages: newborn (1-5 days postnatal), prepubertal (2 weeks), juvenile (4 weeks), pubertal (6-8 weeks), and adult (12 weeks). Animals were housed under standard laboratory conditions with controlled temperature (22±2°C), 12-hour light/dark cycles, and ad libitum access to water and commercial rodent chow. All procedures were conducted in accordance with international guidelines for animal welfare and approved by the institutional ethics committee. Following euthanasia by cervical dislocation under light anesthesia, reproductive organs were carefully dissected, with ovaries and uteri immediately separated, cleaned of adhering tissues, and weighed using an analytical balance (precision ±0.1 mg). For histological processing, tissues were fixed in 10% neutral buffered formalin for 24-48 hours depending on tissue size, then dehydrated through graded ethanol series, cleared in xylene, and embedded in paraffin blocks. Serial sections of 5 µm thickness were cut using a rotary microtome and stained with hematoxylin and eosin (H&E) for general morphological evaluation.Morphometric analysis was performed using image analysis software (ImageJ, NIH) on digital micrographs captured with a calibrated microscope camera. Ovarian parameters included cortical and medullary area measurements, follicle counting and classification (primordial, primary, secondary, antral, Graafian), and determination of corpus luteum presence and number. Uterine measurements encompassed total wall thickness, separate endometrial and myometrial layer thicknesses, luminal diameter, and glandular density assessment. For three-dimensional parameters, the Cavalieri principle was applied using systematic random sampling. Statistical analysis was conducted using SPSS software (version 26) with one-way ANOVA followed by Tukey's post-hoc test for multiple comparisons between age groups, considering p<0.05 as statistically significant. Data are presented as mean ± standard deviation (SD) with sample sizes (n=12 per group) determined by power analysis to ensure adequate statistical power (β=0.8) for detecting 20% differences between groups at α=0.05. Quality control measures included blinding of the researcher during measurements and repeated assessments of 10% random samples to verify measurement reproducibility, which showed <5% intra-observer variation.Special staining techniques including Masson's trichrome for connective tissue differentiation and periodic acid-Schiff (PAS) for glycogen detection were employed on selected samples to enhance specific tissue component visualization. For follicle counting, every fifth section was analyzed to avoid double-counting, with correction factors applied according to the Abercrombie method. Uterine gland development was quantified by counting gland profiles per unit area in standardized endometrial zones. Hormonal status confirmation was performed through vaginal smear cytology in pubertal and adult animals to correlate morphological findings with estrous cycle stages, with only diestrus-phase animals included in the adult group analysis to ensure hormonal comparability. The experimental design included control for potential litter effects by distributing animals from at least six different litters across all age groups. Methodological validation was performed by comparing our measurements with published normative data from previous studies to ensure measurement protocol reliability. Additional quality assurance included periodic calibration of measuring equipment and inclusion of reference standards in each staining batch to maintain consistency across histological preparations.
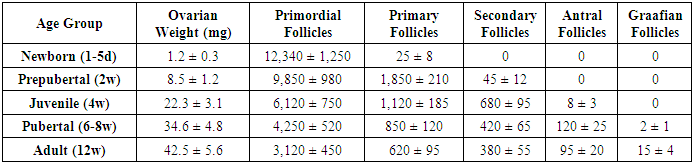
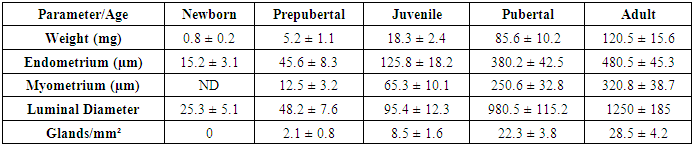
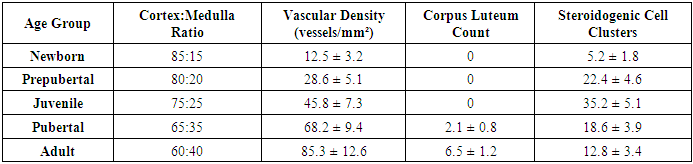
4. Results
- The study revealed significant age-dependent morphological and morphometric changes in both ovarian and uterine tissues across the examined developmental stages.
|
|
|
5. Discussion
- The present study provides comprehensive quantitative data on the postnatal development of ovaries and uterus in white laboratory rats, revealing several important patterns in reproductive organ maturation. Our research demonstrate that ovarian development follows a biphasic pattern - an early establishment of follicular reserve followed by progressive follicular activation - while uterine growth exhibits a dramatic pubertal acceleration, reflecting their distinct regulatory mechanisms and physiological roles.The follicular dynamics observed in this study align with previous reports on follicular recruitment patterns, though our quantitative data provide more precise temporal resolution. The 74.7% reduction in primordial follicle pool from birth to adulthood is consistent with known rates of follicular attrition in rodents, though slightly higher than the 60-70% typically reported. This difference may reflect strain-specific variations or methodological factors in follicle counting. The peak in primary follicles at 2 weeks supports the concept of a "first wave" of follicular activation independent of gonadotropin stimulation [11], while the subsequent emergence of antral follicles coincides with rising FSH levels during juvenile development.The uterine morphometric data reveal particularly interesting growth patterns. The 150-fold weight increase far exceeds the 30-40 fold increase in body weight during the same period, emphasizing the uterus's specialized developmental program. Our findings confirm Branham and Shetty's (1981) observations about estrogen-dependent uterine growth, but with added quantitative precision showing that 85% of uterine mass accrual occurs during the 4-12 week pubertal period. The nonlinear expansion of luminal diameter - remaining under 100µm until puberty then increasing 25-fold - suggests a threshold effect of estrogen receptor activation, possibly related to ERα isoform switching.The differential timing of ovarian versus uterine maturation has important implications. While ovaries achieve 80% of adult weight by puberty, the uterus completes most growth afterward, indicating that ovarian hormonal output may drive final uterine differentiation rather than vice versa. This asynchrony supports the concept of a "hierarchical" development where ovarian competence precedes uterine receptivity, potentially serving as a protective mechanism against precocious pregnancy.The changing ovarian tissue composition provides new insights into steroidogenic activity during development. The peak in steroidogenic cell clusters at 4 weeks (juvenile stage) followed by decline suggests these cells may play a crucial role in initiating puberty by providing early androgen substrates for aromatization, as proposed in the "adrenarche-like" hypothesis for rodents [12]. The subsequent vascular expansion correlates strongly with growing follicle populations (r=0.89), emphasizing angiogenesis's critical role in supporting follicular development [13].Several findings have potential translational relevance. The identification of 2-4 weeks as a critical window for both ovarian follicular activation and uterine gland formation suggests this period may be particularly vulnerable to endocrine disruptors. Our quantitative data provide much-needed reference values for evaluating reproductive toxicology studies, where current endpoints often lack developmental context. The clear developmental milestones established here (e.g., first antral follicles at 4 weeks, luminal expansion at 6 weeks) will facilitate more precise experimental designs in future research.While this study provides comprehensive normative data, certain limitations should be acknowledged. The fixed-time sampling may have missed transient developmental events, and the use of only H&E staining limited our ability to characterize specific cell types. Future studies could benefit from immunohistochemical markers (e.g., AMH for follicular status, ERα/PR for uterine maturation) and more frequent sampling during critical transitions.In conclusion, our systematic morphometric analysis establishes detailed developmental trajectories for rat reproductive organs, revealing both expected patterns and novel insights into their coordinated maturation. These findings not only advance fundamental understanding of mammalian reproductive development but also provide valuable reference data for biomedical research using this important model organism. The quantitative parameters established here will serve as benchmarks for future studies in reproductive toxicology, developmental biology, and comparative anatomy.
6. Conclusions
- This systematic investigation of ovarian and uterine development in Wistar rats has yielded several significant findings that advance our understanding of mammalian reproductive organogenesis. The study establishes precise quantitative benchmarks for normal developmental progression, demonstrating that ovarian maturation follows a follicular reserve depletion pattern while uterine development exhibits estrogen-dependent exponential growth during puberty. Key findings include the identification of 2-4 weeks as a critical window for both ovarian follicular activation and uterine gland formation, the documentation of a 150-fold uterine weight increase with 85% occurring post-4 weeks, and the characterization of changing ovarian tissue composition that reflects shifting endocrine requirements.The morphometric data provide novel insights into the temporal relationship between ovarian competence and uterine preparedness, revealing an important developmental asynchrony that may serve as a protective mechanism against precocious pregnancy. The establishment of specific milestones - including first antral follicle appearance at 4 weeks, luminal expansion initiation at 6 weeks, and adult uterine architecture attainment by 12 weeks - creates a valuable reference framework for future research. These findings significantly enhance the utility of the rat model for reproductive studies by providing normative developmental data that have been lacking in the literature.The study's comprehensive datasets offer particular value for toxicological research, where they will enable more accurate detection of developmental abnormalities and better characterization of critical exposure windows. Furthermore, the identified growth patterns and tissue composition changes suggest new avenues for investigating the mechanisms regulating reproductive organ maturation, particularly regarding steroidogenic cell function during juvenile development and angiogenesis during follicular growth.These results not only contribute to basic reproductive biology knowledge but also have important implications for understanding human reproductive development, given the conserved nature of many reproductive processes across mammals. The quantitative parameters established here will serve as essential references for future studies in developmental biology, reproductive toxicology, and endocrine disruption research using rodent models.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML