-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(5): 1334-1337
doi:10.5923/j.ajmms.20251505.04
Received: Mar. 23, 2025; Accepted: Apr. 21, 2025; Published: May 8, 2025

Cardiotoxity in the Chemotherapy of Breast Cancer
Djuraeva N. O. , Abdurakhmanov M. M. , Abdurakhmanov Z. M.
Bukhara State Medical Institute, Bukhara, Uzbekistan
Correspondence to: Abdurakhmanov Z. M. , Bukhara State Medical Institute, Bukhara, Uzbekistan.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

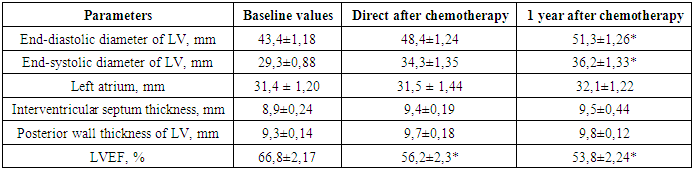
Objective. To study cardiotoxicity arising from the use of anticancer treatment and its impact on the cardiovascular system. Materials and methods. This research investigated patient data from sixty-eight individuals who received outpatient palliative chemotherapy at the Bukhara branch of the Republican Specialized Scientific-Practical Medical Center of Oncology and Radiology for breast cancer (BC). The average age of patients was 54,2±2,2 years (from 34 to 78 years). Of the 68 patients with breast cancer, 58,82% (n=40) had cardiovascular comorbidities, of which arterial hypertension of II stage with left ventricular hypertrophy registered in 70% cases (n=28). The study included patients with stage II and stage III breast cancer. The treatment regimen used was a combination of doxorubicin and cyclophosphamide followed by paclitaxel. All patients underwent the necessary diagnostic tests, including echocardiography and the concentration of natriuretic hormone (B-type) N-terminal propeptide (NT-proBNP) in blood to assess the state of the cardiovascular system. The dynamics of the parameters were assessed before the start of chemotherapy, direct and 1 year after the start of chemotherapy. Results. Immediately after completion of chemotherapy, patients showed a significantly reduced left ventricular ejection fraction (LVEF) of 56,2±2,3. During follow-up one year after completion of polychemotherapy, even lower LVEF values (53,8±2,24%) and increased end-diastolic diameter (51,3±1,26 mm) and end-systolic diameter (36,2±1,33 mm) were determined (p <0,025). During follow-up one year after completion of polychemotherapy, even lower LVEF values (53,8±2,24%) and increased end-diastolic diameter (51,3±1,26 mm) and end-systolic diameter (36,2±1,33 mm) were determined (p <0,025). In addition, the concentration of NT-proBNP in the blood of patients after completion of antitumor polychemotherapy is significantly higher compared to baseline values (112±0,8 pg/ml versus 48,25±0,6 pg/ml, p <0,05). Analysis of the relationship between the level of NT-proBNP concentration and the development of anthracycline cardiotoxicity revealed a significant trend 1 year after chemotherapy, in which NT-proBNP production was six times higher than the initial level (p <0,05), reflecting progressive anthracycline cardiotoxicity. Conclusions. Regular cardiac monitoring is crucial for breast cancer patients undergoing chemotherapy to identify and manage potential heart damage. The results of our study confirm the need to use supportive therapy in the treatment of patients with breast cancer, including immunocorrective, antioxidant and cardioprotective drugs which can help reduce the incidence of cardiotoxicity and improve the quality of life of patients.
Keywords: Cardiotoxity, Cardiovascular system, Breast cancer
Cite this paper: Djuraeva N. O. , Abdurakhmanov M. M. , Abdurakhmanov Z. M. , Cardiotoxity in the Chemotherapy of Breast Cancer, American Journal of Medicine and Medical Sciences, Vol. 15 No. 5, 2025, pp. 1334-1337. doi: 10.5923/j.ajmms.20251505.04.
1. Introduction
- Breast cancer (BC) is a significant medical and social problem and is the predominant oncological pathology in women (20,9%). Improved methods of diagnosis and treatment of breast cancer will lead to an increase in survival rates over the past 20 years: the 5-year survival rate is 89%, 10-year - 82%, 15-year - 77% [5]. The use of modern innovative drugs and more intensive standard chemotherapy regimens has led to an increase in relapse-free survival and life expectancy of patients suffering from cancer. However, the use of certain chemotherapeutic drugs and the introduction of such methods of drug therapy as targeted medication have caused a number of complications, the most serious of which is cardiotoxicity [6]. An example of such a drug is cyclophosphamide in a combination of with chemotherapeutic drugs, especially anthracycline antibiotics, is accompanied by an increase in the manifestation of cardiotoxicity [8,19]. Of note, the fatality rate associated with cardiomyopathy ranges from 2 to 17% among individuals undergone therapy with cyclophosphamide [2].The published preliminary results of the ESC-COT (Cardiac-Oncology Toxicity) EACVI/HFA Pilot registry showed left ventricular dysfunction during the follow-up in 2,9% patients received a targeted therapy against breast cancer. Thus, patients receiving cardiotoxic chemotherapy should undergo a cardiological examination during the follow-up after completion of treatment [9]. The aim of the study was to identify cardiotoxity in patients receiving targeted therapy for breast cancer, especially the use of a combination of doxorubicin and cyclophosphamide followed by paclitaxel.
2. Materials and Methods
- The sample size was 68 patients who received outpatient palliative chemotherapy at the Bukhara branch of the Republican Specialized Scientific-Practical Medical Center of Oncology and Radiology for BC. The use of monoclonal antibodies in healthcare practice has become available recently, so the number of observations was limited. Informed consent was obtained from the patients.The average age of patients was 54,2±2,2 years (from 34 to 78 years). The prevalence of the tumor was estimated in accordance with the 7th edition of the TNM classification (UICC, 2009). Most patients had stage II (31 patients) or stage III (23 patients) of breast cancer.Of the 68 patients with breast cancer, 58,82% (40 women) had cardiovascular pathologies before the start of treatment. In the structure of these pathologies, hypertension accounted for 77,5% (31 women) of the total number of diseases. It is important to note that most patients (70%) had arterial hypertension of II stage with heart damage in the form of left ventricular hypertrophy. In seven patients, hypertension was combined with ischemic heart disease, and three patients had chronic rheumatic heart disease.The treatment regimen used was a combination of doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 administered every three weeks followed by paclitaxel (100 mg/m2) over 3 h every 3 weeks. Four courses of chemotherapy were administered according to both regimens. The cumulative dose of doxorubicin for this group ranged from 300 to 360 mg/m2.All patients underwent the necessary diagnostic tests, including echocardiography and NT-proBNP parameter to assess the state of intracardiac hemodynamics. Measurements of interventricular septal and posterior wall thickness and intramural LV dimensions were determined using parasternal long-axis imaging of the LV. These linear measurements were made both directly in B-mode and in M-mode. The dynamics of the parameters were assessed before, direct and 12 months after the start of chemotherapy. During statistical data processing, an assessment was made of the parameters’ belonging to the normal distribution. If the parameters corresponded to the normal distribution, they were described by the mean value (M) and standard deviation (SD). Quantitative parameters were assessed using the Student’s t-test for dependent variables. Differences between parameters were considered statistically significant at a two-sided p value <0,05.
3. Results
- Before analyzing the obtained results of the study, it should be noted that all the measured parameters remained within the established normal range throughout the entire observation period. In this regard, the present study only allows us to point out some of the trends found with a limited degree of reliability due to the small amount of data and adherence to a strict experimental protocol. After completion of the polychemotherapy course, changes associated with depression of the global systolic function of the left ventricle were expectedly recorded, which was manifested in a change in its geometry. These changes were characterized by an increase in the volume of the ventricular cavity and an increase in the relative thickness of its walls.Immediately after completion of chemotherapy, patients showed a significantly reduced LVEF of 56,2 ± 2,3 (Table 1).
|
|
4. Discussion
- Numerous studies have documented that the primary factor limiting the dosage of cyclophosphamide is its potential to cause cardiac damage. This cardiotoxicity has only been observed following administration of high doses of cyclophosphamide. This condition can present as a severe form of inflammation affecting the heart muscle and surrounding tissue, potentially leading to hemorrhagic necrotic perimyocarditis due to the direct endothelial cells damage, interstitial hemorrhage and edema. This serious complication carries a high risk of sudden cardiac arrest and death [1,12]. Doxorubicin’s cardiotoxic effect, such as nitrosative, oxydative stress, mytochondrial dysfunction and inflammation are also acknowledged in the literature that also emphasizes their potential in improving cardiomyopathy for breast cancer patients [15,20]. Studies have shown that treatment with cyclophosphamide and doxorubicin leads to a substantial reduction in LVEF in a significant proportion of patients, ranging from 4,7% to 48% [3,4,17]. Cancer therapy-related cardiac dysfunction is generally characterized by a decrease in LVEF of at least 10%, falling below 50% [18]. In our study, we found a tendency of LVEF decline within 1 year after cyclophosphamide therapy, on the contrary LVEDD and LVESD increased what are probably associated with intracellular damage caused by increased oxidative stress. Myocardial relaxation based on the function of microfilaments and the sarcoplasmic calcium pump requires energy, which may be insufficient under the pathological effects of oxidation products.The study aimed at analyzing the relationship between the level of brain natriuretic peptide expression and manifestations of anthracycline-induced cardiotoxicity showed that a significant increase in the expression of this peptide in patients with cardiotoxic myocardial remodeling is a result of predominant NT-proBNP production [16]. This fact seems promising for use as a prognostic marker for assessing the degree of cardiotoxicity progression, as well as for monitoring the effectiveness of preventive therapy. Determining the concentration of NT-proBNP in the blood provides an opportunity for reliable stratification of the risks of anthracycline cardiotoxicity and myocardial damage at the initial stages of asymptomatic cardiac dysfunction [7]. This is important given that such manifestations of heart failure as dyspnea, fatigue and peripheral edema usually become noticeable after significant apoptosis of cardiomyocytes and subsequent deterioration of the myocardium.
5. Conclusions
- Myocardial dysfunction and heart failure are serious manifestations of cancer treatment-induced cardiotoxicity. For timely detection of cardiac complications, it is necessary to continuously monitor the functional properties of the heart during chemotherapy treatment, as well as after completion of chemotherapy by means of echocardiography and study of NT-proBNP concentration in blood plasma. This can simplify to identify patients with a high risk of cardiotoxic complications and mortality, which necessitates more intensive and individualized optimization of pathogenetic therapy. The results of our study confirmed the necessity and validity of using supportive therapy in the treatment of patients with breast cancer, including immunocorrective, antioxidant and cardioprotective drugs which significantly could improve the effectiveness of systemic chemotherapy using anthracyclines reducing the incidence of cardiotoxicity and improving the quality of life of patients [10,11,13,14].In conclusion, it is noted that further research should be aimed at better understanding the mechanisms of cardiotoxicity and adopting the preventive cardioprotective therapy. Long-term clinical trials with a large number of patients are needed to determine the most effective strategies for preventing and managing cardiotoxicity, including cardioprotective drugs, the use of which can improve the effectiveness of systemic chemotherapy using anthracyclines, help reduce the incidence of cardiotoxicity with an improvement in the quality of life of patients. It is also important to conduct training and exchange experiences between specialists in immunology, cardiology and oncology in order to create multispecialty teams capable of effectively responding to the risks of cardiotoxicity and providing the best treatment for patients with cancer.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML