-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(5): 1331-1333
doi:10.5923/j.ajmms.20251505.03
Received: Mar. 19, 2025; Accepted: Apr. 21, 2025; Published: May 8, 2025

Evaluation of Cardiac Remodeling After Myocardial Infarction in Patients with Type 2 Diabetes
Rakhimov Khikmatullo Khusanboevich
"Republican Specialized Scientific and Practical Medical Center of Therapy and Medical Rehabilitation", Tashkent, Uzbekistan
Correspondence to: Rakhimov Khikmatullo Khusanboevich , "Republican Specialized Scientific and Practical Medical Center of Therapy and Medical Rehabilitation", Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The article provides data on the study of Echocardiographic features of changes in the left ventricle after myocardial infarction in diabetic type 2 patients. The purpose of the study is to determine whether clinical and Echocardiographic data are associated with the clinical course of the disease. The study involved 96 patients aged 52.52 and 6.21 in Type 2 myocardial infarction diabetes patients undergoing inpatient treatment at the Republican Specialized Scientific and Practical Medical Center of Therapy and Medical Rehabilitation. Diabetes Mellitus Type 2 was directly related to the clinical condition assessment scale indicator in patients with myocardial infarction and the six-minute walk test and reverse correlation with LVEF.
Keywords: Diabetes mellitus, Myocardial infarction, Echocardiography
Cite this paper: Rakhimov Khikmatullo Khusanboevich , Evaluation of Cardiac Remodeling After Myocardial Infarction in Patients with Type 2 Diabetes, American Journal of Medicine and Medical Sciences, Vol. 15 No. 5, 2025, pp. 1331-1333. doi: 10.5923/j.ajmms.20251505.03.
Article Outline
1. Introduction
- Cardiac remodeling is the result of a complex interaction between neurohormonal and cytokine signaling cascades, which, by a positive feedback mechanism, stimulate the processes of cell structural rearrangement leading to ventricular remodeling [1,2]. These include infiltration by inflammatory mono nuclear cells and proliferation of fibroblasts, some of which can differentiate into contractile myofibroblasts, stimulation of myocyte hypertrophy and an increase in the rate of myocyte apoptosis. This complex of cellular changes ultimately leads to characteristic morphological changes in the left ventricle, including dilatation and progressive LV dysfunction. LVH occurs as a result of exposure to pathological factors, and it is aimed at maintaining the normal function of the organ - at maintaining cardiac output (CO), which is determined by the efficiency of its systolic and diastolic functions. It is well known that the fall in CO with a decrease in myocardial contractile function is compensated by two mechanisms: Frank-Star ling and activation of neurohumoral systems: activation of the SAS, RAAS and the arginine-vasopressin system leads to an increase in vascular tone, sodium and water retention with an increase in circulating blood volume, which triggers a long-term compensation mechanism, the main component of which is remodeling [2,3]. In type 2 diabetes mellitus (DM2), it is the heart that is recognized as the most important target of damage. The combination of insulin resistance, hyperinsulinemia and hyperglycemia accelerates the development and progression of diseases associated with atherosclerosis, increasing the risk of cardiovascular complications [4]. Left ventricle (LV) diastolic dysfunction is considered an early marker of myocardial damage in T2DM, noting the pathogenetic relationship between LV diastolic dysfunction and LV hypertrophy (LVH) in the development and progression of chronic heart failure (CHF) [5]. Despite the available material on the pathogenesis of remodeling, the results of the work do not reflect a complete picture of the processes of formation of structural and functional disorders of the heart in patients with type 2 diabetes in CHF. The question of the severity of heart changes in electrophysiological remodeling in T2DM remains unresolved.
2. Objective
- To evaluate the processes of cardiac remodeling in patients with type 2 diabetes mellitus (DM) after myocardial infarction (MI).
3. Purpose of Work
- The purpose of the study is to determine the relationship of clinical data to the clinical course of the disease, to optimize the prognosis of post-myocardial infarction remodeling in diabetic type 2 patients.
4. Methods
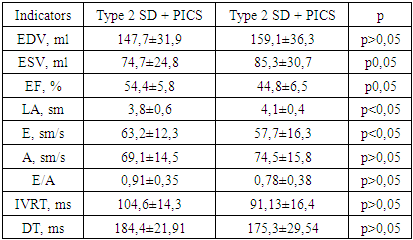
- The study included 97 male patients with MI at the age of 40 to 60 years (mean age 51.3±5.7 years). The survey included patients with a history of MI from 6 months to 3 years. To compare the data obtained, a group of healthy individuals (control group) was examined in the amount of 31 people comparable in sex and age with the main group. The structural and functional state of the myocardium and the process of LV remodeling was assessed by Doppler echocardiography. Echocardiography was carried out on the MEDISON ACCUVIX V20 device (Korea), using a 3.25 MHz probe in standard echocardiographic positions, by the transthoracic method in the supine position and on the left side by EchoCG in M- and B-modes in accordance with the recommendations of the American Association of Echocardiography (ASE). During echocardiography, the structural parameters of the heart were assessed: LV end-diastolic volume (EDV), LV end-systolic volume (ESV), thickness of left ventricular posterior wall (TLVPPW), interventricular septal thickness (IST), fraction of shortening of the anteroposterior LV size in systole (Fs), the size of the left atrium (LA), the longitudinal size of the left ventricle, defined as the distance from the base of the papillary muscles to the apex of the heart in systole and diastole (Ls and Ld). The value of the mean hemodynamic BP (AP mean) was calculated using the Hi Kem formula. End systolic volume (ESV), end diastolic volume (EDV), ejection fraction (EF) were calculated based on the obtained data using the Simpson formula. Evaluation of LV diastolic function by determining indicators: the maximum rate of early filling of the left ventricle (E), the maximum rate of late filling of the atria (A), the ratio of E / A, DT is the time of slowing down the flow rate in the early LV filling phase (ms), isovolumetric relaxation time left ventricle (IRTLV, ms).
5. Results
- When evaluating the parameters of postinfarction left ventricular (LV) remodeling in the examined patients, the following types of remodeling were identified: 28 (29.5%) had a concentric type of remodeling, 36 (37.9%) had concentric LV hypertrophy and an eccentric type of remodeling - in 31 (32.6%) patients. Disorders of LV diastolic function were observed in 86 (90.5%) patients, of which relaxation disorders were determined in 41 (47.7%) cases, pseudonormalization - in 27 (31.4%) and restrictive changes in 18 (20.9%) cases.
|
6. Discussion
- Imaging techniques, especially echocardiography, are necessary for the recognition of preclinical left ventricular (LV) diastolic disturbances, as well as further tracking of pathological changes and responses to treatment [1]. In patients with a modern treatment of MI, diabetes mellitus remains a major and independent predictor of subsequent heart failure. This higher risk is not associated with a decreased left-ventricular systolic function or with increased left-ventricular remodeling. The evidence of higher left-ventricular filling pressures suggests left-ventricular diastolic dysfunction as a potential mechanism [4]. Diabetes interact synergistically to influence cardiac remodeling. These findings may explain the markedly heightened risk of heart failure and cardiovascular disease [5].
7. Conclusions
- In patients with type 2 diabetes who have had myocardial infarction, post-infarction remodeling leads not only to structural restructuring of the left ventricle, characterized by a decrease in myocardial contractility, a change in the structural and geometric parameters of the heart, but also to severe LV diastolic dysfunction, which is a prognostically significant factor in the formation of heart failure.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML