Ashurov Zarifjon Sharifovich 1, Tadjibaev Uktam Askarovich 2, Khayredinova Inara Ilgizovna 3, Ibragimova Saida Yunusovna 4, Ravshanov Jakhongir Azimjon Ugli 5, Abdukakharova Gulnoza Kurbanovna 6
1DSc, Professor, Department Psychiatry and Narcology, Tashkent Medical Academy, Tashkent, Uzbekistan
2PhD Сandidate, Clinical Department for the Treatment of Alcoholism, Republican Specialized Scientific-Practical Medical Center of Mental Health, Tashkent Region, Uzbekistan
3PhD, Senior Lecturer, Department Psychiatry and Narcology, Tashkent Medical Academy, Tashkent, Uzbekistan
4Medical Doctor, Сlinical and Diagnostic Department, Republican Specialized Scientific-Practical Medical Center of Mental Health, Tashkent Region, Uzbekistan
5Assistant, Department Psychiatry and Narcology, Tashkent Medical Academy, Tashkent, Uzbekistan
6PhD Сandidate, Clinical Department for the Treatment of Drug Addiction and Substance Abuse, Republican Specialized Scientific-Practical Medical Center of Mental Health, Tashkent Region, Uzbekistan
Correspondence to: Tadjibaev Uktam Askarovich , PhD Сandidate, Clinical Department for the Treatment of Alcoholism, Republican Specialized Scientific-Practical Medical Center of Mental Health, Tashkent Region, Uzbekistan.
| Email: |  |
Copyright © 2025 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Chronic alcohol dependence affects neurophysiological processes, often leading to electroencephalographic (EEG) abnormalities. Traumatic brain injury (TBI) further exacerbates these changes, increasing seizure risk. Additionally, severe COVID-19 may contribute to long-term EEG alterations. However, limited research has explored the combined impact of alcohol dependence, TBI, and severe COVID-19 on EEG patterns. This study aims to analyze EEG changes in patients with these conditions to identify seizure risk factors. Methods: This study included 110 patients with a history of TBI, divided into two groups based on withdrawal severity: 39 patients with severe withdrawal syndrome and 71 with a moderate form. Additionally, a subgroup of 22 patients with a history of severe COVID-19 was identified as an additional risk factor. EEG recordings were analyzed for rhythm slowing, epileptiform activity, and seizure threshold indices. ANOVA and Pearson’s correlation were used for statistical analysis. Results: Rhythm slowing was observed in 45%, epileptiform activity in 30%, and a mixed pattern in 25%. Severe withdrawal was associated with increased epileptiform activity and a lower seizure threshold (p < 0.01). TBI and COVID-19 further amplified EEG abnormalities. Conclusions: Alcohol dependence, TBI, and COVID-19 collectively increase seizure risk. EEG abnormalities correlated with withdrawal severity, alcohol use duration, and prior COVID-19 infection, emphasizing the need for early diagnosis and seizure risk monitoring.
Keywords:
Chronic alcohol dependence, Traumatic brain injury, Alcohol withdrawal syndrome, Electroencephalogram (EEG), Seizures, COVID-19, Neurophysiological impairment
Cite this paper: Ashurov Zarifjon Sharifovich , Tadjibaev Uktam Askarovich , Khayredinova Inara Ilgizovna , Ibragimova Saida Yunusovna , Ravshanov Jakhongir Azimjon Ugli , Abdukakharova Gulnoza Kurbanovna , EEG Monitoring in Assessing Seizure Risk in Patients with Alcohol Dependence, Traumatic Brain Injury, and Severe COVID-19: Integrated Statistical Analysis and Practical Recommendations, American Journal of Medicine and Medical Sciences, Vol. 15 No. 4, 2025, pp. 1217-1222. doi: 10.5923/j.ajmms.20251504.76.
1. Introduction
Chronic alcohol dependence significantly impacts neurophysiological processes, as evidenced by electroencephalographic (EEG) studies. Porjesz and Begleiter (2003) demonstrated that individuals with alcohol dependence exhibit alterations in EEG rhythmicity and power, indicating an imbalance between excitatory and inhibitory processes in the brain [1]. Quantitative analysis of the alpha rhythm has also revealed significant differences in patients with alcohol dependence. Galkin et al. (2022) identified a correlation between changes in the alpha rhythm and the progression of withdrawal syndrome [2]. These findings highlight the importance of EEG in monitoring the condition of patients with alcohol dependence. Traumatic brain injury (TBI) is an additional risk factor for seizures in individuals with alcohol dependence. The use of invasive recording methods, such as electrocorticography, enables more precise detection of epileptiform patterns compared to scalp EEG [3]. Clinical studies confirm that severe withdrawal syndrome increases the risk of seizures, making it a critical issue in both addiction medicine and neurology [4,5]. Another factor influencing brain function in this patient population is a history of severe COVID-19. Campanella et al. (2009) reported that neuroinflammation caused by severe COVID-19 can exacerbate neurophysiological impairments, further increasing the risk of seizures [6]. Automated EEG analysis methods are also promising in diagnosing alcohol dependence. Acharya et al. (2014) demonstrated that computerized EEG analysis provides high accuracy in detecting alcohol dependence-related changes [7]. Furthermore, biofeedback therapy (EEG-based neurofeedback) has shown effectiveness in reducing alcohol cravings and stress levels, making it a promising method for comprehensive alcohol dependence treatment [8].Thus, modern research confirms the importance of comprehensive EEG analysis in diagnosing and monitoring patients with chronic alcohol dependence. The study of additional factors, such as TBI and COVID-19, is particularly relevant for understanding seizure risks in this patient population.The study aims to identify patterns in EEG changes in 110 patients with stage II alcohol dependence, all of whom have a history of traumatic brain injury (TBI).
2. Materials and Methods
Sample Selection and Inclusion CriteriaThis study analyzed electroencephalographic (EEG) data from 110 patients diagnosed with stage II alcohol dependence, all of whom had a documented history of traumatic brain injury (TBI). The selection of patients was based on the following criteria:Age Range: Participants were between 20 and 60 years old.Diagnosis: Alcohol dependence was confirmed according to the international classification criteria (ICD-10).History of TBI: All patients had a medically documented history of traumatic brain injury, ranging from mild to moderate severity, with corresponding neurological manifestations.Alcohol Withdrawal Syndrome: Clinical evaluation confirmed that all patients exhibited signs of alcohol withdrawal syndrome. Based on the severity of withdrawal symptoms, the study sample was divided into two groups: Group 1: Patients with severe withdrawal syndrome (n = 39).Group 2: Patients with moderate withdrawal syndrome (n = 71).Additionally, among the total study cohort, 22 patients (20%) had experienced a severe course of COVID-19. These cases were distributed between the groups as follows: 12 patients in Group 1 and 10 patients in Group 2.By analyzing EEG patterns in this specific population, the study aims to uncover potential neurophysiological changes that could inform clinical decision-making and risk assessment for seizure development in patients with coexisting conditions of alcohol dependence, TBI, and severe COVID-19.Collection of Clinical DataFor each patient, a comprehensive set of clinical data was gathered to ensure a thorough assessment of their neurological and medical history. The collected information included:• History of Alcohol Abuse: This covered the duration of alcohol misuse (in years), frequency of consumption, and the quantity of alcohol consumed.• Traumatic Brain Injury (TBI) Data: The nature of the injury, findings from computed tomography (CT) and/or magnetic resonance imaging (MRI), as well as neurological symptoms documented in the patient’s medical history were recorded.• Assessment of Alcohol Withdrawal Syndrome: The severity of withdrawal symptoms was evaluated using standardized clinical scales, such as the Clinical Institute Withdrawal Assessment for Alcohol-Revised (CIWA-Ar), which allowed for an objective differentiation of symptom severity.• COVID-19 History: Severe cases of COVID-19 were confirmed through medical documentation, including records of hospitalization, laboratory test results, and other relevant clinical findings.EEG Examination ProtocolAll electroencephalography (EEG) recordings were conducted under standardized clinical conditions to ensure the reliability of the data. The examination followed a structured protocol:• Patient Condition: EEG recordings were performed during a state of relaxed wakefulness, under fixed environmental conditions, including a controlled room setting with consistent lighting and minimal background noise.•Equipment and Methodology: ο A 16-channel electroencephalograph was used, with electrode placement following the international 10-20 system to ensure standardized data collection.ο Recordings were conducted with both open and closed eyes to assess rhythm reactivity.ο Key EEG parameters analyzed included absolute spectral power in the alpha, theta, and delta frequency bands, as well as the presence of epileptiform patterns, such as spike-wave discharges and peak-pattern activity.• Recording Duration: Each EEG session lasted at least 30 minutes, ensuring sufficient data for detecting rhythmic and periodic patterns, which are critical for evaluating seizure risk.• Data Processing: Spectral analysis software was used to process EEG data, alongside visual interpretation by an experienced clinical neurophysiologist, providing a detailed assessment of brain activity patterns.Statistical AnalysisTo ensure a comprehensive evaluation of the collected data, various statistical methods were applied:• Descriptive Statistics: Mean values, standard deviations, and percentage distributions were calculated to characterize the clinical parameters and EEG patterns observed in the study population.• One-Way Analysis of Variance (ANOVA): This method was used to compare the mean duration of alcohol misuse and levels of seizure activity between the two groups—patients with severe withdrawal syndrome and those with moderate withdrawal symptoms.• Correlation Analysis (Pearson’s Correlation Coefficient): The relationship between the severity of traumatic brain injury (TBI), the intensity of alcohol withdrawal syndrome, and the level of epileptiform activity was examined to identify potential associations.Ethical ConsiderationsAll collected data were fully anonymized to protect patient confidentiality, and the study was conducted in strict accordance with the ethical principles outlined in the Declaration of Helsinki. Prior to participation, all patients provided informed consent, allowing their medical data to be used for scientific research.
3. Results and Discussion
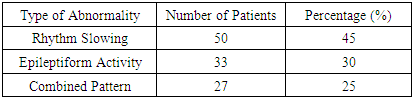
Distribution of EEG PatternsThe overall analysis of EEG recordings from the 110 patients revealed distinct patterns of brain activity:• 45% (50 patients) exhibited pronounced rhythm slowing, characterized by an increase in theta and delta waves and a reduction in alpha rhythm.• 30% (33 patients) showed a predominance of epileptiform activity, including spike-wave discharges and peak-pattern activity.• 25% (27 patients) demonstrated a combination of both alterations, presenting a mixed pattern of EEG abnormalities.These findings highlight the variability in neurophysiological changes among patients with alcohol dependence and a history of traumatic brain injury, reinforcing the need for individualized approaches to seizure risk assessment.Table 1. Distribution of EEG Patterns in 110 Patients
 |
| |
|
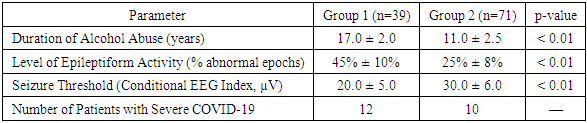
The key differences in clinical parameters between the two groups are presented in Table 2.Table 2. Comparison of Clinical Parameters Between Groups
 |
| |
|
Group 1 (patients with a longer history of alcohol abuse) exhibited significantly more pronounced neurological impairments. These included a higher level of epileptiform activity, a lower seizure threshold, and more severe consequences of alcohol misuse.A direct correlation was observed between the duration of alcohol abuse and the worsening of neurological status, indicating an increased risk of seizures and other neurological complications. This underscores the critical importance of early detection and intervention in chronic alcoholism to prevent severe neurological consequences.3. One-Way Analysis of Variance (ANOVA)The one-way analysis of variance (ANOVA) identified statistically significant differences between the groups:Duration of Alcohol Abuse: Significant differences were found between the groups (F = 5.3, p < 0.01).Level of Epileptiform Activity: A notable difference was observed (F = 6.1, p < 0.01).Correlation AnalysisFurther correlation analysis using Pearson’s correlation coefficient revealed strong positive associations:Severity of TBI and Level of Epileptiform Activity (r = 0.62, p < 0.01).Severity of Alcohol Withdrawal Syndrome and Lowered Seizure Threshold (r = 0.58, p < 0.01).Impact of Severe COVID-19 on EEG PatternsAdditionally, the analysis of severe COVID-19 cases demonstrated that patients with a history of severe COVID-19 exhibited more pronounced EEG abnormalities compared to those without such a history. This finding suggests that severe COVID-19 may serve as an independent predictor of increased seizure risk.DiscussionKey EEG Abnormalities in Severe and Moderate Alcohol Withdrawal SyndromeAlcohol withdrawal syndrome is associated with characteristic EEG changes, the severity of which correlates with the intensity of withdrawal symptoms. In patients with moderate withdrawal syndrome, EEG typically shows increased beta activity and reduced alpha rhythm power compared to normal values. These findings reflect a state of neuronal hyperexcitability due to alcohol cessation. However, the overall rhythmic organization remains relatively intact, with no significant disorganization—meaning that EEG in moderate withdrawal often appears near-normal, aside from these subtle alterations.In contrast, severe withdrawal syndrome (especially during delirium tremens) presents with more pronounced EEG abnormalities. A diffuse slowing of rhythms is frequently observed, marked by an increase in theta waves and a reduction in alpha activity, indicating cortical dysfunction and encephalopathic suppression. However, despite this, central nervous system hyperexcitability persists, creating a paradoxical combination of neuronal overactivation and cognitive deficits. Notably, quantitative EEG (QEEG) analysis has revealed that after alcohol-related seizures, all patients exhibit increased fast beta power and reduced alpha power. Interestingly, among those who later developed delirium, the relative power of high-frequency β3 rhythms in the frontal leads was lower, while the theta/alpha ratio was significantly higher compared to patients who did not develop delirium. In other words, as withdrawal progresses toward delirium, the EEG signal becomes lower in frequency and more disorganized, reflecting widespread cortical dysfunction and autonomic dysregulation.Thus, while moderate alcohol withdrawal is primarily characterized by increased frequency and decreased amplitude of background rhythms, severe withdrawal is marked by the onset of generalized encephalopathy, as evident in EEG recordings.Impact of Chronic Alcohol Abuse on EEG ParametersLong-term alcohol abuse leads to persistent functional changes in the brain, which are detectable on EEG. Numerous studies have reported that chronic alcoholics exhibit suppressed resting alpha rhythms and a relative increase in both slower and faster background components. For instance, a study by Galkin et al. (2022) found a statistically significant reduction in spectral power of the alpha band across all neocortical areas in patients with alcohol dependence compared to healthy individuals. Furthermore, the microstructure of alpha spindles was altered, with a predominance of a flattened or low-amplitude alpha rhythm and higher oscillation frequency. This suggests that the normal dominant resting rhythm (8–12 Hz) in alcoholics becomes weaker and more disorganized, aligning with the theory of excitatory-inhibitory imbalance in the CNS caused by chronic alcohol intoxication.Importantly, the duration and severity of alcoholism are directly correlated with the extent of EEG abnormalities. Reduced alpha rhythm power is associated with a faster progression of alcohol dependence and an earlier onset of alcohol use. Moreover, decreased alpha reactivity (i.e., less suppression of alpha activity upon eye opening) correlates with longer withdrawal episodes, stronger cravings for alcohol, and a more aggressive course of dependence. In other words, the longer and more intense the alcohol abuse, the more pronounced the objective signs of brain dysfunction on EEG. Additionally, chronic alcoholism leads to cortical atrophy and neural network dysfunction, as confirmed by both neuroimaging studies and the progressive decline in EEG rhythm amplitudes with increasing years of alcohol use. Ultimately, long-term alcohol abuse creates a pathological EEG profile characterized by persistent alpha suppression, increased beta/theta activity, and overall cortical deactivation.Severity of Alcohol Withdrawal Syndrome, Epileptiform Activity, and Seizure ThresholdSevere alcohol withdrawal syndrome significantly increases the risk of seizures, reflecting a lowered seizure threshold. According to large-scale clinical studies, approximately 10% of patients undergoing alcohol withdrawal experience generalized seizures (so-called alcohol-related seizures) within the first 1–2 days after stopping alcohol consumption. This highlights how intense neuronal hyperexcitability and neurotransmitter imbalance during withdrawal can overcome normal inhibitory mechanisms, leading to seizures.However, routine EEG recordings outside of seizure episodes do not always reveal epileptiform activity in these patients. In typical cases of alcohol-related seizures (not associated with structural epilepsy), interictal EEG findings may appear relatively normal, aside from a diffuse reduction in the amplitude of the dominant rhythm. The absence of clear epileptiform discharges or focal slowing in patients with withdrawal seizures suggests that the seizures are primarily due to alcohol withdrawal rather than an underlying epileptogenic brain lesion. Conversely, if an EEG taken outside of a seizure episode reveals prominent abnormalities, the presence of an underlying structural epilepsy should be considered.In the most severe forms of withdrawal—especially in alcohol withdrawal delirium (delirium tremens)—EEG is almost always abnormal. This is often characterized by diffuse slowing and bursts of dysrhythmias, indicating an extremely low seizure threshold. It is also well documented that withdrawal seizures themselves predispose patients to developing delirium, making them a potential early warning sign of more severe withdrawal syndromes. The reverse is also true: a history of delirium tremens significantly increases the likelihood of recurrent seizures during future withdrawal episodes.This phenomenon aligns with the kindling mechanism, where each successive withdrawal episode tends to be more severe than the last, and the risk of seizure complications progressively increases. As a result, patients with multiple past withdrawal episodes represent a high-risk group for epileptiform reactions.Overall, greater withdrawal severity is associated with more frequent epileptiform EEG abnormalities and a lower seizure threshold. While isolated withdrawal seizures may occur with minimal EEG changes, their presence signals severe alcohol-induced neurotoxicity and underscores the urgent need for intensive treatment to prevent progression to delirium and status epilepticus.Impact of Past COVID-19 Infection on Neurophysiological ParametersA growing body of evidence suggests that severe COVID-19 can lead to long-term alterations in brain function, including changes in EEG patterns. In patients who have recovered from severe COVID-19—especially those who experienced encephalopathy, mechanical ventilation (MV), and other critical conditions—EEG abnormalities are almost always observed in the acute phase.A systematic review and meta-analysis found that approximately 96% of COVID-19 patients who underwent EEG exhibited pathological background activity. The most frequent finding was diffuse slowing, characterized by global suppression of the normal alpha rhythm, loss of reactivity, and overall desynchronization—a pattern commonly associated with metabolic or hypoxic encephalopathy. Additionally, around 20% of patients with COVID-19-related encephalopathy displayed epileptiform discharges (such as spikes and sharp waves), indicating cortical irritability. However, clinical seizures or status epilepticus were relatively rare, occurring in only 2–3% of cases, according to EEG monitoring. Thus, while severe COVID-19 rarely triggers overt epilepsy, it almost always induces encephalopathic EEG changes, reflecting central nervous system involvement.Long-Term Neurophysiological Effects of COVID-19 ("Long-COVID")Several studies have documented the persistence of neurophysiological abnormalities and associated cognitive impairments in the long-term phase of COVID-19 recovery. Even in cases of mild to moderate COVID-19, some patients exhibit reduced electrical activity in frontal brain regions and a simplification of EEG signals 6 to 12 months post-infection. Specifically, research has shown a significant decrease in wave power in the Fz–F4 region during resting states and cognitive load tasks in post-COVID patients compared to healthy controls. Additionally, reduced fractal complexity and variability of EEG signals in frontal-parietal leads have been reported, which may indicate long-term impairment of functional neural connectivity. Clinically, these changes correlate with the phenomenon known as "brain fog"—a cluster of symptoms including memory impairment, attention deficits, and slowed thinking reported by many COVID-19 survivors.Thus, COVID-19 exerts a multi-level impact on the brain:• In the acute phase, it causes transient diffuse dysfunction, evident on EEG as rhythm slowing and, in some cases, epileptiform activity.• In the long term, it leads to more subtle neurophysiological alterations, which are closely linked to cognitive difficulties in post-COVID patients.These findings highlight the need for long-term neurological follow-up in patients recovering from severe COVID-19 to better understand and manage its lasting effects on brain function.Comparison of Findings with Previous Research on Alcoholism, TBI, and COVID-19The patterns identified in our study are generally consistent with previous research and expand the existing understanding of the combined effects of alcoholism, traumatic brain injury (TBI), and past COVID-19 infection on brain function. It is well established that chronic alcoholism leads to characteristic EEG changes. As early as Porjesz and Begleiter (2003), studies demonstrated that the EEG of individuals with alcohol dependence differs significantly from that of healthy individuals, reflecting a disrupted balance between excitation and inhibition in the central nervous system (CNS). Our findings, which show reduced alpha activity and increased beta oscillations in alcohol-dependent patients, align with these observations. Additionally, the association between withdrawal severity and EEG rhythm slowing that we identified is consistent with QEEG data. For example, Yoon et al. (2022) found that the progression to delirium during alcohol withdrawal is accompanied by increased theta activity and a relative reduction in beta activity on EEG. This supports our conclusion that severe withdrawal syndrome leads to widespread disorganization of the brain’s bioelectrical activity.Regarding traumatic brain injury (TBI), our observations confirm the well-known exacerbating effect of alcoholism on traumatic brain damage. Previous research by Rönty et al. (1993) demonstrated that alcohol-dependent patients with TBI exhibit larger intracranial hemorrhages, more pronounced atrophic changes on CT scans, and a slower recovery as measured by quantitative EEG (QEEG) compared to non-drinking TBI patients. In our study, patients with both alcoholism and TBI also showed more persistent EEG abnormalities over time, which is in line with these earlier findings. Thus, the combination of chronic alcohol use and TBI results in cumulative damage, leading to more severe and long-lasting neurophysiological impairments than would be expected from either condition alone.The impact of COVID-19 on EEG in our study sample also mirrors trends described in the literature. Nearly all patients who had severe COVID-19 exhibited diffuse background activity abnormalities, including EEG slowing and the disappearance of the alpha rhythm, findings consistent with meta-analytical data (≈96% of cases with abnormal background activity). Additionally, we observed isolated epileptiform phenomena (sporadic sharp waves) in some patients, which corresponds to the ~20% frequency of such findings reported in other studies.Overall, our findings do not contradict previously published data but rather complement them, offering a unique perspective by analyzing EEG changes in a patient group affected by all three risk factors—alcohol dependence, TBI, and severe COVID-19. To date, only limited research has explored the impact of COVID-19 on individuals with alcohol dependence or TBI. Our study is among the first to present a comprehensive analysis of neurophysiological alterations in alcohol-dependent patients affected by both TBI and COVID-19, highlighting the multifaceted impact of these interacting factors on brain function.
4. Conclusions
The study results demonstrated significant neurophysiological impairments in patients with stage II alcohol dependence who had a history of traumatic brain injury (TBI). Duration of alcohol abuse directly correlates with worsening EEG parameters: Patients with a longer history of alcoholism exhibited a greater reduction in seizure threshold, increased epileptiform activity, and disorganization of background rhythms, all of which increase the risk of seizure complications. Severity of alcohol withdrawal syndrome is associated with alterations in brain bioelectrical activity: Patients with severe withdrawal symptoms showed a higher frequency of epileptiform discharges and generalized EEG slowing, indicating profound cortical dysfunction. TBI exacerbates the neurophysiological consequences of alcoholism: Patients with both TBI and prolonged alcohol abuse exhibited more persistent and pronounced EEG abnormalities, confirming the additive effect of brain damage in these conditions. Severe COVID-19 is an additional risk factor: Patients with a history of severe COVID-19 demonstrated greater abnormalities in background EEG activity and an increased tendency toward epileptiform reactions. This finding underscores the need for further research on the impact of viral infections on neurophysiology in individuals with substance dependence. Practical Implications. These findings highlight the critical need for early diagnosis and intensive treatment of alcohol withdrawal syndrome, particularly in patients with a history of TBI and COVID-19. Increased vigilance regarding seizure risks in this patient group should be a priority in developing treatment strategies for alcohol dependence.
References
| [1] | B. Porjesz and H. Begleiter, “Alcoholism and human electrophysiology,” Alcohol Res. Health, vol. 27, no. 2, pp. 153–160, 2003. |
| [2] | S. A. Galkin, N. I. Kisel, A. I. Mandel, and N. A. Bokhan, “Quantitative characteristics of the alpha-band of the electroencephalogram in people with alcohol dependence,” Zh. Nevrol. Psikhiatr. Im. S. S. Korsakova, vol. 122, no. 5, pp. 105–110, 2022, doi: 10.17116/jnevro2022122051105. |
| [3] | H. Zhang, J. Yao, C. Xu, and C. Wang, “Targeting electroencephalography for alcohol dependence: A narrative review,” CNS Neurosci. Ther., vol. 29, no. 5, pp. 1205–1212, May 2023, doi: 10.1111/cns.14138. |
| [4] | U. R. Acharya, S. V, S. Bhat, H. Adeli, and A. Adeli, “Computer-aided diagnosis of alcoholism-related EEG signals,” Epilepsy Behav., vol. 41, pp. 257–263, Dec. 2014, doi: 10.1016/j.yebeh.2014.10.001. |
| [5] | K. H. M. Lima, J. S. Gomes, and A. M. Tucci, “Electroencephalographic neurofeedback as a tool for reducing harm and risk associated with alcohol use disorder: A critical review,” Drug Alcohol Rev., vol. 41, no. 3, pp. 594–602, Mar. 2022, doi: 10.1111/dar.13387. |
| [6] | S. Campanella, G. Petit, P. Maurage, C. Kornreich, P. Verbanck, and X. Noël, “Chronic alcoholism: insights from neurophysiology,” Neurophysiol. Clin., vol. 39, no. 4–5, pp. 191–207, Oct.–Nov. 2009, doi: 10.1016/j.neucli.2009.08.002. |
| [7] | E. S. Freedland and D. B. McMicken, “Alcohol-related seizures, Part I: Pathophysiology, differential diagnosis, and evaluation,” J. Emerg. Med., vol. 11, no. 4, pp. 463–473, Jul.–Aug. 1993, doi: 10.1016/0736-4679(93)90251-2. |
| [8] | T. M. Sokhadze, R. L. Cannon, and D. L. Trudeau, “EEG biofeedback as a treatment for substance use disorders: review, rating of efficacy, and recommendations for further research,” Appl. Psychophysiol. Biofeedback, vol. 33, no. 1, pp. 1–28, Mar. 2008, doi: 10.1007/s10484-007-9047-5. |


 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML
